Responses in Rat Core Auditory Cortex are Preserved during Sleep Spindle Oscillations
- PMID: 26856904
- PMCID: PMC4835306
- DOI: 10.5665/sleep.5758
Responses in Rat Core Auditory Cortex are Preserved during Sleep Spindle Oscillations
Abstract
Study objectives: Sleep is defined as a reversible state of reduction in sensory responsiveness and immobility. A long-standing hypothesis suggests that a high arousal threshold during non-rapid eye movement (NREM) sleep is mediated by sleep spindle oscillations, impairing thalamocortical transmission of incoming sensory stimuli. Here we set out to test this idea directly by examining sensory-evoked neuronal spiking activity during natural sleep.
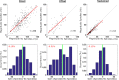
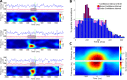
Methods: We compared neuronal (n = 269) and multiunit activity (MUA), as well as local field potentials (LFP) in rat core auditory cortex (A1) during NREM sleep, comparing responses to sounds depending on the presence or absence of sleep spindles.
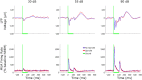
Results: We found that sleep spindles robustly modulated the timing of neuronal discharges in A1. However, responses to sounds were nearly identical for all measured signals including isolated neurons, MUA, and LFPs (all differences < 10%). Furthermore, in 10% of trials, auditory stimulation led to an early termination of the sleep spindle oscillation around 150-250 msec following stimulus onset. Finally, active ON states and inactive OFF periods during slow waves in NREM sleep affected the auditory response in opposite ways, depending on stimulus intensity.
Conclusions: Responses in core auditory cortex are well preserved regardless of sleep spindles recorded in that area, suggesting that thalamocortical sensory relay remains functional during sleep spindles, and that sensory disconnection in sleep is mediated by other mechanisms.
Keywords: LFP; NREM sleep; auditory cortex; rat; single-unit; sleep spindles.
© 2016 Associated Professional Sleep Societies, LLC.
Figures





Similar articles
-
Sleep Differentially Affects Early and Late Neuronal Responses to Sounds in Auditory and Perirhinal Cortices.J Neurosci. 2020 Apr 1;40(14):2895-2905. doi: 10.1523/JNEUROSCI.1186-19.2020. Epub 2020 Feb 18. J Neurosci. 2020. PMID: 32071140 Free PMC article.
-
Auditory responses and stimulus-specific adaptation in rat auditory cortex are preserved across NREM and REM sleep.Cereb Cortex. 2015 May;25(5):1362-78. doi: 10.1093/cercor/bht328. Epub 2013 Dec 8. Cereb Cortex. 2015. PMID: 24323498 Free PMC article.
-
The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats.Eur J Neurosci. 2009 Mar;29(5):1071-81. doi: 10.1111/j.1460-9568.2009.06654.x. Epub 2009 Feb 24. Eur J Neurosci. 2009. PMID: 19245368
-
Spontaneous neural activity during human non-rapid eye movement sleep.Prog Brain Res. 2011;193:111-8. doi: 10.1016/B978-0-444-53839-0.00008-9. Prog Brain Res. 2011. PMID: 21854959 Review.
-
Neuronal oscillations in sleep: insights from functional neuroimaging.Neuromolecular Med. 2012 Sep;14(3):154-67. doi: 10.1007/s12017-012-8166-1. Epub 2012 Jan 25. Neuromolecular Med. 2012. PMID: 22274804 Review.
Cited by
-
The Degree of Nesting between Spindles and Slow Oscillations Modulates Neural Synchrony.J Neurosci. 2020 Jun 10;40(24):4673-4684. doi: 10.1523/JNEUROSCI.2682-19.2020. Epub 2020 May 5. J Neurosci. 2020. PMID: 32371605 Free PMC article.
-
Pupil Size Coupling to Cortical States Protects the Stability of Deep Sleep via Parasympathetic Modulation.Curr Biol. 2018 Feb 5;28(3):392-400.e3. doi: 10.1016/j.cub.2017.12.049. Epub 2018 Jan 18. Curr Biol. 2018. PMID: 29358069 Free PMC article.
-
Neurophysiological features of STN LFP underlying sleep fragmentation in Parkinson's disease.J Neurol Neurosurg Psychiatry. 2024 Nov 18;95(12):1112-1122. doi: 10.1136/jnnp-2023-331979. J Neurol Neurosurg Psychiatry. 2024. PMID: 38724231 Free PMC article.
-
Sleep restores an optimal computational regime in cortical networks.Nat Neurosci. 2024 Feb;27(2):328-338. doi: 10.1038/s41593-023-01536-9. Epub 2024 Jan 5. Nat Neurosci. 2024. PMID: 38182837 Free PMC article.
-
Absent sleep EEG spindle activity in GluA1 (Gria1) knockout mice: relevance to neuropsychiatric disorders.Transl Psychiatry. 2018 Aug 14;8(1):154. doi: 10.1038/s41398-018-0199-2. Transl Psychiatry. 2018. PMID: 30108203 Free PMC article.
References
-
- Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. St. Louis, MO: Saunders/Elsevier; 2011. pp. 16–26.
-
- Rechtschaffen A, Hauri P, Zeitlin M. Auditory awakening thresholds in REM and NREM sleep stages. Percept Mot Skills. 1966;22:927–42. - PubMed
-
- Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16:467–77. - PubMed
-
- Rechtschaffen A. The single-mindedness and isolation of dreams. Sleep. 1978;1:97–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

