CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis
- PMID: 26856995
- PMCID: PMC5232416
- DOI: 10.1007/112_2015_5002
CFTR: A New Horizon in the Pathomechanism and Treatment of Pancreatitis
Abstract
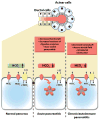
Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel that conducts chloride and bicarbonate ions across epithelial cell membranes. Mutations in the CFTR gene diminish the ion channel function and lead to impaired epithelial fluid transport in multiple organs such as the lung and the pancreas resulting in cystic fibrosis. Heterozygous carriers of CFTR mutations do not develop cystic fibrosis but exhibit increased risk for pancreatitis and associated pancreatic damage characterized by elevated mucus levels, fibrosis, and cyst formation. Importantly, recent studies demonstrated that pancreatitis causing insults, such as alcohol, smoking, or bile acids, strongly inhibit CFTR function. Furthermore, human studies showed reduced levels of CFTR expression and function in all forms of pancreatitis. These findings indicate that impairment of CFTR is critical in the development of pancreatitis; therefore, correcting CFTR function could be the first specific therapy in pancreatitis. In this review, we summarize recent advances in the field and discuss new possibilities for the treatment of pancreatitis.
Keywords: CFTR; Cystic fibrosis; Epithelial transport; Pancreas; Pancreatitis.
Conflict of interest statement
the authors have no conflict of interest to declare
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

