Durable Benefits of Cellular Postconditioning: Long-Term Effects of Allogeneic Cardiosphere-Derived Cells Infused After Reperfusion in Pigs with Acute Myocardial Infarction
- PMID: 26857066
- PMCID: PMC4802479
- DOI: 10.1161/JAHA.115.002796
Durable Benefits of Cellular Postconditioning: Long-Term Effects of Allogeneic Cardiosphere-Derived Cells Infused After Reperfusion in Pigs with Acute Myocardial Infarction
Abstract
Background: Infusion of allogeneic cardiosphere-derived cells (allo-CDCs) postreperfusion elicits cardioprotective cellular postconditioning in pigs with acute myocardial infarction. However, the long-term effects of allo-CDCs have not been assessed. We performed a placebo-controlled pivotal study for long-term evaluation, as well as shorter-term mechanistic studies.
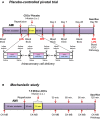
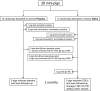
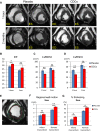
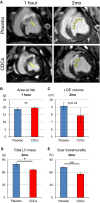
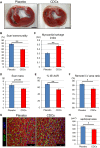
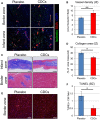
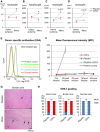
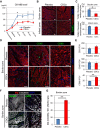
Methods and results: Minipigs underwent 1.5-hour mid-left anterior descending balloon occlusion followed by reperfusion and were randomized to receive intracoronary allo-CDCs or vehicle 30 minutes postreperfusion. Left ventriculography (LVG) demonstrated preserved ejection fraction (EF) and attenuation of LV remodeling in CDC-treated pigs. Pigs underwent cardiac magnetic resonance imaging (MRI) and LVG 1 hour and 8 weeks after therapy to evaluate efficacy. MRI showed improvement of EF and attenuation of LV remodeling immediately after allo-CDC infusion. In addition, allo-CDCs improved regional function and decreased hypertrophy 2 months post-treatment. Histological analysis revealed increased myocardial salvage index, enhanced vascularity, sustained reductions in infarct size/area at risk and scar transmurality, and attenuation of collagen deposition in the infarct zone of allo-CDC-treated pigs at 2 months. Allo-CDCs did not evoke lymphohistiocytic infiltration or systemic humoral memory response. Short-term experiments designed to probe mechanism revealed antiapoptotic effects of allo-CDCs on cardiomyocytes and increases in cytoprotective macrophages, but no increase in overall inflammatory cell infiltration 2 hours after cell therapy.
Conclusions: Allo-CDC infusion postreperfusion is safe, improves cardiac function, and attenuates scar size and remodeling. The favorable effects persist for at least 2 months after therapy. Thus, cellular postconditioning confers not only acute cardioprotection, but also lasting structural and functional benefits.
Keywords: allogeneic transplantation; cardioprotective effect; cardiosphere‐derived cells.
© 2016 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures

References
-
- Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–286. - PubMed
-
- Kanazawa H, Tseliou E, Malliaras K, Yee K, Dawkins JF, De Couto G, Smith RR, Kreke M, Seinfeld J, Middleton RC, Gallet R, Cheng K, Luthringer D, Valle I, Chowdhury S, Fukuda K, Makkar RR, Marban L, Marban E. Cellular postconditioning: allogeneic cardiosphere‐derived cells reduce infarct size and attenuate microvascular obstruction when administered after reperfusion in pigs with acute myocardial infarction. Circ Heart Fail. 2015;8:322–332. - PMC - PubMed
-
- Malliaras K, Smith RR, Kanazawa H, Yee K, Seinfeld J, Tseliou E, Dawkins JF, Kreke M, Cheng K, Luthringer D, Ho CS, Blusztajn A, Valle I, Chowdhury S, Makkar RR, Dharmakumar R, Li D, Marban L, Marban E. Validation of contrast‐enhanced magnetic resonance imaging to monitor regenerative efficacy after cell therapy in a porcine model of convalescent myocardial infarction. Circulation. 2013;128:2764–2775. - PMC - PubMed
-
- Swindle MM. Swine in the Laboratory: Surgery, Anesthesia, Imaging and Experimental Techniques. Boca Raton, FL: CRC Press; 2007.
-
- Payne AR, Casey M, McClure J, McGeoch R, Murphy A, Woodward R, Saul A, Bi X, Zuehlsdorff S, Oldroyd KG, Tzemos N, Berry C. Bright‐blood T2‐weighted MRI has higher diagnostic accuracy than dark‐blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ Cardiovasc Imaging. 2011;4:210–219. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

