Properties of an optogenetic model for olfactory stimulation
- PMID: 26857095
- PMCID: PMC4929330
- DOI: 10.1113/JP271853
Properties of an optogenetic model for olfactory stimulation
Abstract
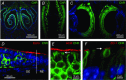
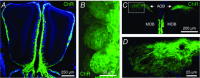
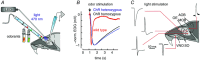
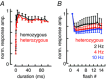
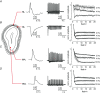
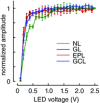
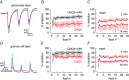
Key points: In olfactory research it is difficult to deliver stimuli with defined intensity and duration to olfactory sensory neurons. Expression of channelrhodopsin 2 (ChR2) in olfactory sensory neurons provides a means to activate these neurons with light flashes. Appropriate mouse models are available. The present study explores the suitability of an established olfactory marker protein (OMP)/ChR2-yellow fluorescent protein (YFP) mouse model for ex vivo experimentation. Expression of ChR2 in sensory neurons of the main olfactory epithelium, the septal organ and vomeronasal organ is characterized. Expression pattern of ChR2 in olfactory receptor neurons and the properties of light responses indicate that light stimulation does not impact on signal transduction in the chemosensory cilia. Light-induced electro-olfactograms are characterized with light flashes of different intensities, durations and frequencies. The impact of light-induced afferent stimulation on the olfactory bulb is examined with respect to response amplitude, polarity and low-pass filtering.
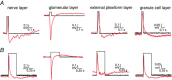
Abstract: For the examination of sensory processing, it is helpful to deliver stimuli in precisely defined temporal and spatial patterns with accurate control of stimulus intensity. This is challenging in experiments with the mammalian olfactory system because airborne odorants have to be transported into the intricate sensory structures of the nose and must dissolve in mucus to be detected by sensory neurons. Defined and reproducible activity can be generated in olfactory sensory neurons that express the light-gated ion channel channelrhodopsin 2 (ChR2). The neurons can be stimulated by light flashes in a controlled fashion by this optogenetic approach. Here we examined the application of an olfactory marker protein (OMP)/ChR2-yellow fluorescent protein (YFP) model for ex vivo exploration of the olfactory epithelium and the olfactory bulb of the mouse. We studied the expression patterns of ChR2 in the main olfactory system, the vomeronasal system, and the septal organ, and we found that ChR2 is absent from the sensory cilia of olfactory sensory neurons. In the olfactory epithelium, we characterized light-induced electro-olfactograms with respect to peripheral encoding of stimulus intensity, stimulus duration and stimulus frequency. In acute slices of the olfactory bulb, we identified specific aspects of the ChR2-induced input signal, concerning its dynamic range, its low-pass filter property and its response to prolonged stimulation. Our study describes the performance of the OMP/ChR2-YFP model for ex vivo experimentation on the peripheral olfactory system and documents its versatility and its limitations for olfactory research.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures
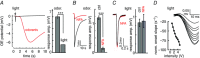

References
-
- Alonso M, Lepousez G, Sebastien W, Bardy C, Gabellec MM, Torquet N & Lledo PM (2012). Activation of adult‐born neurons facilitates learning and memory. Nat Neurosci 15, 897–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

