Cooling-induced SUMOylation of EXOSC10 down-regulates ribosome biogenesis
- PMID: 26857222
- PMCID: PMC4793216
- DOI: 10.1261/rna.054411.115
Cooling-induced SUMOylation of EXOSC10 down-regulates ribosome biogenesis
Abstract
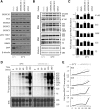
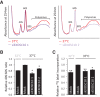
The RNA exosome is essential for 3' processing of functional RNA species and degradation of aberrant RNAs in eukaryotic cells. Recent reports have defined the substrates of the exosome catalytic domains and solved the multimeric structure of the exosome complex. However, regulation of exosome activity remains poorly characterized, especially in response to physiological stress. Following the observation that cooling of mammalian cells results in a reduction in 40S:60S ribosomal subunit ratio, we uncover regulation of the nuclear exosome as a result of reduced temperature. Using human cells and an in vivo model system allowing whole-body cooling, we observe reduced EXOSC10 (hRrp6, Pm/Scl-100) expression in the cold. In parallel, both models of cooling increase global SUMOylation, leading to the identification of specific conjugation of SUMO1 to EXOSC10, a process that is increased by cooling. Furthermore, we define the major SUMOylation sites in EXOSC10 by mutagenesis and show that overexpression of SUMO1 alone is sufficient to suppress EXOSC10 abundance. Reducing EXOSC10 expression by RNAi in human cells correlates with the 3' preribosomal RNA processing defects seen in the cold as well as reducing the 40S:60S ratio, a previously uncharacterized consequence of EXOSC10 suppression. Together, this work illustrates that EXOSC10 can be modified by SUMOylation and identifies a physiological stress where this regulation is prevalent both in vitro and in vivo.
Keywords: 40S subunits; RNA exosome; SUMOylation; cold shock; rRNA processing.
© 2016 Knight et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





Similar articles
-
Hypoxia-driven deSUMOylation of EXOSC10 promotes adaptive changes in the transcriptome profile.Cell Mol Life Sci. 2024 Jan 27;81(1):58. doi: 10.1007/s00018-023-05035-9. Cell Mol Life Sci. 2024. PMID: 38279024 Free PMC article.
-
The ubiquitin-specific protease USP36 SUMOylates EXOSC10 and promotes the nucleolar RNA exosome function in rRNA processing.Nucleic Acids Res. 2023 May 8;51(8):3934-3949. doi: 10.1093/nar/gkad140. Nucleic Acids Res. 2023. PMID: 36912080 Free PMC article.
-
EXOSC10/Rrp6 is essential for the eight-cell embryo/morula transition.Dev Biol. 2022 Mar;483:58-65. doi: 10.1016/j.ydbio.2021.12.010. Epub 2021 Dec 26. Dev Biol. 2022. PMID: 34965385
-
Regulation of the conserved 3'-5' exoribonuclease EXOSC10/Rrp6 during cell division, development and cancer.Biol Rev Camb Philos Soc. 2021 Aug;96(4):1092-1113. doi: 10.1111/brv.12693. Epub 2021 Feb 18. Biol Rev Camb Philos Soc. 2021. PMID: 33599082 Review.
-
SUMO routes ribosome maturation.Nucleus. 2011 Nov-Dec;2(6):527-32. doi: 10.4161/nucl.2.6.17604. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064470 Review.
Cited by
-
Helicase-Dependent RNA Decay Illuminated by a Cryo-EM Structure of a Human Nuclear RNA Exosome-MTR4 Complex.Cell. 2018 Jun 14;173(7):1663-1677.e21. doi: 10.1016/j.cell.2018.05.041. Cell. 2018. PMID: 29906447 Free PMC article.
-
SUMO pathway is required for ribosome biogenesis.BMB Rep. 2022 Nov;55(11):535-540. doi: 10.5483/BMBRep.2022.55.11.130. BMB Rep. 2022. PMID: 36195568 Free PMC article. Review.
-
Hypoxia-driven deSUMOylation of EXOSC10 promotes adaptive changes in the transcriptome profile.Cell Mol Life Sci. 2024 Jan 27;81(1):58. doi: 10.1007/s00018-023-05035-9. Cell Mol Life Sci. 2024. PMID: 38279024 Free PMC article.
-
PD-L1 (B7-H1) Competes with the RNA Exosome to Regulate the DNA Damage Response and Can Be Targeted to Sensitize to Radiation or Chemotherapy.Mol Cell. 2019 Jun 20;74(6):1215-1226.e4. doi: 10.1016/j.molcel.2019.04.005. Epub 2019 Apr 30. Mol Cell. 2019. PMID: 31053471 Free PMC article.
-
Tissue-Resident-Memory CD8+ T Cells Bridge Innate Immune Responses in Neighboring Epithelial Cells to Control Human Genital Herpes.Front Immunol. 2021 Sep 6;12:735643. doi: 10.3389/fimmu.2021.735643. eCollection 2021. Front Immunol. 2021. PMID: 34552595 Free PMC article.
References
-
- Al-Fageeh MB, Marchant RJ, Carden MJ, Smales CM. 2006. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol Bioeng 93: 829–835. - PubMed
-
- Becker J, Barysch SV, Karaca S, Dittner C, Hsiao HH, Berriel Diaz M, Herzig S, Urlaub H, Melchior F. 2013. Detecting endogenous SUMO targets in mammalian cells and tissues. Nat Struct Mol Biol 20: 525–531. - PubMed
-
- Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, Fujita J. 1997. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 236: 804–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/M501773/1/MRC_/Medical Research Council/United Kingdom
- MC_U132692719/MRC_/Medical Research Council/United Kingdom
- BB/I020055/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F018908/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F018738/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
