Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule
- PMID: 26857443
- PMCID: PMC4819841
- DOI: 10.3109/13697137.2015.1136926
Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule
Abstract
Objective: To evaluate the bioavailability and safety of a novel vaginal capsule containing solubilized bioidentical 17β-estradiol for vulvar and vaginal atrophy and compare its pharmacokinetics with that of an approved vaginal estradiol tablet in healthy postmenopausal women.
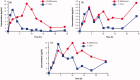
Methods: Two randomized, single-dose, two-way cross-over, relative bioavailability trials compared the pharmacokinetics of a solubilized vaginal estradiol softgel capsule (TX-004HR, test) with that of a vaginal estradiol tablet (Vagifem®, reference) in postmenopausal women (aged 40-65 years) at 10-μg and 25-μg doses. In each study, women were randomly assigned to receive a single dose of the test capsule or reference tablet, followed by a single dose of the alternate drug after a 14-day washout.
Results: Thirty-five women completed the 10-μg study and 36 completed the 25-μg study. Significantly lower systemic levels of estradiol, estrone, and estrone sulfate at both doses of the test product were observed compared with equivalent doses of the reference product, with lower AUC0-24 and Cmax and earlier tmax. No adverse events were reported in either trial.
Conclusion: TX-004HR, a novel estradiol vaginal softgel capsule, exhibited significantly lower systemic exposure than equivalent doses of an approved vaginal estradiol tablet at both 10-μg and 25-μg doses. Both doses of each product were safe and well-tolerated.
Keywords: Bioavailability; estradiol; menopause; pharmacokinetics; vaginal estrogen therapy; vulvar and vaginal atrophy.
Figures

References
-
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6:2133–42. - PubMed
-
- Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the Vaginal Health: Insights, Views & Attitudes survey. Menopause. 2013;20:1043–8. - PubMed
-
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10:1790–9. - PubMed
-
- Management of symptomatic vulvovaginal atrophy 2013 position statement of The North American Menopause Society. Menopause. 2013;20:888–902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
