Immunopathology of Japanese macaque encephalomyelitis is similar to multiple sclerosis
- PMID: 26857488
- PMCID: PMC4748211
- DOI: 10.1016/j.jneuroim.2015.11.026
Immunopathology of Japanese macaque encephalomyelitis is similar to multiple sclerosis
Abstract
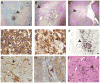
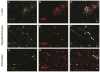
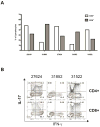
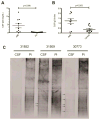
Japanese macaque encephalomyelitis (JME) is an inflammatory demyelinating disease that occurs spontaneously in a colony of Japanese macaques (JM) at the Oregon National Primate Research Center. Animals with JME display clinical signs resembling multiple sclerosis (MS), and magnetic resonance imaging reveals multiple T2-weighted hyperintensities and gadolinium-enhancing lesions in the central nervous system (CNS). Here we undertook studies to determine if JME possesses features of an immune-mediated disease in the CNS. Comparable to MS, the CNS of animals with JME contain active lesions positive for IL-17, CD4+ T cells with Th1 and Th17 phenotypes, CD8+ T cells, and positive CSF findings.
Keywords: Demyelination; Inflammatory; Interleukin 17 (IL-17); Intrathecal IgG; Magnetic resonance imaging (MRI); Th1; Th17.
Copyright © 2015 Elsevier B.V. All rights reserved.
Conflict of interest statement
TCB, MM, SDR, KF, IT, JP, and SGK have no competing interests. WDR received grants from Vertex Pharmaceuticals. LSS, DNB and SWW each received research support from the National Multiple Sclerosis Society (NMSS).
Figures

References
-
- BABBE H, ROERS A, WAISMAN A, LASSMANN H, GOEBELS N, HOHLFELD R, FRIESE M, SCHRODER R, DECKERT M, SCHMIDT S, RAVID R, RAJEWSKY K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. - PMC - PubMed
-
- BAETEN DL, KUCHROO VK. How Cytokine networks fuel inflammation: Interleukin-17 and a tale of two autoimmune diseases. Nat Med. 2013;19:824–825. - PubMed
-
- BERER K, WEKERLE H, KRISHNAMOORTHY G. B cells in spontaneous autoimmune diseases of the central nervous system. Mol Immunol. 2011;48:1332–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

