Expression of multiple Bacillus subtilis genes is controlled by decay of slrA mRNA from Rho-dependent 3' ends
- PMID: 26857544
- PMCID: PMC4838369
- DOI: 10.1093/nar/gkw069
Expression of multiple Bacillus subtilis genes is controlled by decay of slrA mRNA from Rho-dependent 3' ends
Abstract
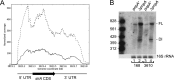
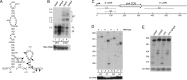
Timely turnover of RNA is an important element in the control of bacterial gene expression, but relatively few specific targets of RNA turnover regulation are known. Deletion of the Bacillus subtilis pnpA gene, encoding the major 3' exonuclease turnover enzyme, polynucleotide phosphorylase (PNPase), was shown previously to cause a motility defect correlated with a reduced level of the 32-gene fla/che flagellar biosynthesis operon transcript.fla/che operon transcript abundance has been shown to be inhibited by an excess of the small regulatory protein, SlrA, and here we find that slrA mRNA accumulated in the pnpA-deletion mutant. Mutation of slrA was epistatic to mutation of pnpA for the motility-related phenotype. Further, Rho-dependent termination was required for PNPase turnover of slrA mRNA. When the slrA gene was provided with a Rho-independent transcription terminator, gene regulation was no longer PNPase-dependent. Thus we show that the slrA transcript is a direct target of PNPase and that regulation of RNA turnover is a major determinant of motility gene expression. The interplay of specific transcription termination and mRNA decay mechanisms suggests selection for fine-tuning of gene expression.
© The Author(s) 2016. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Polynucleotide phosphorylase and RNA helicase CshA cooperate in Bacillus subtilis mRNA decay.RNA Biol. 2021 Nov;18(11):1692-1701. doi: 10.1080/15476286.2020.1864183. Epub 2020 Dec 31. RNA Biol. 2021. PMID: 33323028 Free PMC article.
-
Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain.J Bacteriol. 1996 Apr;178(8):2375-82. doi: 10.1128/jb.178.8.2375-2382.1996. J Bacteriol. 1996. PMID: 8636041 Free PMC article.
-
Global analysis of mRNA decay intermediates in Bacillus subtilis wild-type and polynucleotide phosphorylase-deletion strains.Mol Microbiol. 2014 Oct;94(1):41-55. doi: 10.1111/mmi.12748. Epub 2014 Aug 21. Mol Microbiol. 2014. PMID: 25099370 Free PMC article.
-
Regulatory interplay between small RNAs and transcription termination factor Rho.Biochim Biophys Acta Gene Regul Mech. 2020 Jul;1863(7):194546. doi: 10.1016/j.bbagrm.2020.194546. Epub 2020 Mar 23. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 32217107 Review.
-
Regulation and functions of bacterial PNPase.Wiley Interdiscip Rev RNA. 2016 Mar-Apr;7(2):241-58. doi: 10.1002/wrna.1328. Epub 2016 Jan 11. Wiley Interdiscip Rev RNA. 2016. PMID: 26750178 Review.
Cited by
-
The potential of cold-shock promoters for the expression of recombinant proteins in microbes and mammalian cells.J Genet Eng Biotechnol. 2022 Dec 29;20(1):173. doi: 10.1186/s43141-022-00455-9. J Genet Eng Biotechnol. 2022. PMID: 36580173 Free PMC article. Review.
-
Identification of Genes Required for Swarming Motility in Bacillus subtilis Using Transposon Mutagenesis and High-Throughput Sequencing (TnSeq).J Bacteriol. 2022 Jun 21;204(6):e0008922. doi: 10.1128/jb.00089-22. Epub 2022 May 31. J Bacteriol. 2022. PMID: 35638827 Free PMC article.
-
Regulatory 3' Untranslated Regions of Bacterial mRNAs.Front Microbiol. 2017 Jul 10;8:1276. doi: 10.3389/fmicb.2017.01276. eCollection 2017. Front Microbiol. 2017. PMID: 28740488 Free PMC article. Review.
-
Mastering the control of the Rho transcription factor for biotechnological applications.Appl Microbiol Biotechnol. 2021 May;105(10):4053-4071. doi: 10.1007/s00253-021-11326-7. Epub 2021 May 8. Appl Microbiol Biotechnol. 2021. PMID: 33963893 Review.
-
Comprehensive transcription terminator atlas for Bacillus subtilis.Nat Microbiol. 2022 Nov;7(11):1918-1931. doi: 10.1038/s41564-022-01240-7. Epub 2022 Oct 3. Nat Microbiol. 2022. PMID: 36192538 Free PMC article.
References
-
- Lehnik-Habrink M., Schaffer M., Mader U., Diethmaier C., Herzberg C., Stulke J. RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol. Microbiol. 2011;81:1459–1473. - PubMed
-
- Daou-Chabo R., Mathy N., Benard L., Condon C. Ribosomes initiating translation of the hbs mRNA protect it from 5′-to-3′ exoribonucleolytic degradation by RNase J1. Mol. Microbiol. 2009;71:1538–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

