Trypanosoma cruzi Causes Paralyzing Systemic Necrotizing Vasculitis Driven by Pathogen-Specific Type I Immunity in Mice
- PMID: 26857570
- PMCID: PMC4807493
- DOI: 10.1128/IAI.01497-15
Trypanosoma cruzi Causes Paralyzing Systemic Necrotizing Vasculitis Driven by Pathogen-Specific Type I Immunity in Mice
Abstract
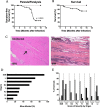
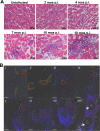
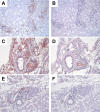
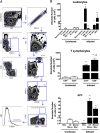
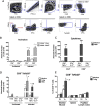
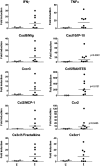
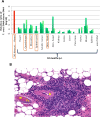
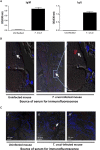
Infectious agents are often considered potential triggers of chronic inflammatory disease, including autoimmunity; however, direct evidence is usually lacking. Here we show that following control of acute infection of mice with the myotropic Colombiana strain of Trypanosoma cruzi, parasites persisted in tissue at low levels associated with development of systemic necrotizing vasculitis. Lesions occurred in many but not all organs and tissues, with skeletal muscle arteries being the most severely affected, and were associated with myositis, atrophy, paresis/paralysis, and death. Histopathology showed fibrinoid vascular necrosis, rare amastigote nests within skeletal muscle myocytes, and massive leukocyte infiltrates composed mainly of inflammatory monocytes, F4/80(+)macrophages, and T. cruzi tetramer-specific CD8(+) T lymphocytes capable of producing gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) but not interleukin-17 (IL-17). T. cruzi-specific IgG was detected in sera from infected mice, but antibody deposits and neutrophilic inflammation were not features of the lesions. Thus,T. cruzi infection of mice may be a specific infectious trigger of paralyzing systemic necrotizing vasculitis most severely affecting skeletal muscle, driven by pathogen-specific type I immune responses.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Low-Level Parasite Persistence Drives Vasculitis and Myositis in Skeletal Muscle of Mice Chronically Infected with Trypanosoma cruzi.Infect Immun. 2019 May 21;87(6):e00081-19. doi: 10.1128/IAI.00081-19. Print 2019 Jun. Infect Immun. 2019. PMID: 30936158 Free PMC article.
-
CCL3/Macrophage Inflammatory Protein-1α Is Dually Involved in Parasite Persistence and Induction of a TNF- and IFNγ-Enriched Inflammatory Milieu in Trypanosoma cruzi-Induced Chronic Cardiomyopathy.Front Immunol. 2020 Mar 3;11:306. doi: 10.3389/fimmu.2020.00306. eCollection 2020. Front Immunol. 2020. PMID: 32194558 Free PMC article.
-
Cavia porcellus as a model for experimental infection by Trypanosoma cruzi.Am J Pathol. 2011 Jul;179(1):281-8. doi: 10.1016/j.ajpath.2011.03.043. Epub 2011 May 14. Am J Pathol. 2011. PMID: 21703410 Free PMC article.
-
[Role of cytokines in resistance and pathology in Trypanosoma cruzi infection].Rev Argent Microbiol. 1996 Apr-Jun;28(2):99-109. Rev Argent Microbiol. 1996. PMID: 8768488 Review. Spanish.
-
[Inflammatory response to acute Trypanosoma cruzi infection].Medicina (B Aires). 1997;57(2):227-34. Medicina (B Aires). 1997. PMID: 9532835 Review. Spanish.
Cited by
-
Pathology and Pathogenesis of Chagas Heart Disease.Annu Rev Pathol. 2019 Jan 24;14:421-447. doi: 10.1146/annurev-pathol-020117-043711. Epub 2018 Oct 24. Annu Rev Pathol. 2019. PMID: 30355152 Free PMC article. Review.
-
Does Autoimmunity Play a Role in the Immunopathogenesis of Vasculitis Associated With Chronic Chagas Disease?Front Cell Infect Microbiol. 2021 Jul 6;11:671962. doi: 10.3389/fcimb.2021.671962. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34295833 Free PMC article. No abstract available.
-
Low-Level Parasite Persistence Drives Vasculitis and Myositis in Skeletal Muscle of Mice Chronically Infected with Trypanosoma cruzi.Infect Immun. 2019 May 21;87(6):e00081-19. doi: 10.1128/IAI.00081-19. Print 2019 Jun. Infect Immun. 2019. PMID: 30936158 Free PMC article.
-
Dynamics of Trypanosoma cruzi infection in hamsters and novel association with progressive motor dysfunction.PLoS Negl Trop Dis. 2024 Jun 21;18(6):e0012278. doi: 10.1371/journal.pntd.0012278. eCollection 2024 Jun. PLoS Negl Trop Dis. 2024. PMID: 38905323 Free PMC article.
-
A novel nanoluciferase-based system to monitor Trypanosoma cruzi infection in mice by bioluminescence imaging.PLoS One. 2018 Apr 19;13(4):e0195879. doi: 10.1371/journal.pone.0195879. eCollection 2018. PLoS One. 2018. PMID: 29672535 Free PMC article.
References
-
- Gonzalez Cappa SM, Sanz OP, Muller LA, Molina HA, Fernandez J, Rimoldi MT, Sica RE. 1987. Peripheral nervous system damage in experimental chronic Chagas' disease. Am J Trop Med Hyg 36:41–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

