Porphyromonas gingivalis Outer Membrane Vesicles Induce Selective Tumor Necrosis Factor Tolerance in a Toll-Like Receptor 4- and mTOR-Dependent Manner
- PMID: 26857578
- PMCID: PMC4807478
- DOI: 10.1128/IAI.01390-15
Porphyromonas gingivalis Outer Membrane Vesicles Induce Selective Tumor Necrosis Factor Tolerance in a Toll-Like Receptor 4- and mTOR-Dependent Manner
Abstract
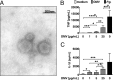
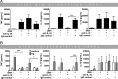
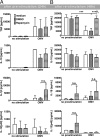
Porphyromonas gingivalis is an important member of the anaerobic oral flora. Its presence fosters growth of periodontal biofilm and development of periodontitis. In this study, we demonstrated that lipophilic outer membrane vesicles (OMV) shed from P. gingivalis promote monocyte unresponsiveness to live P. gingivalis but retain reactivity to stimulation with bacterial DNA isolated from P. gingivalis or AIM2 ligand poly(dA·dT). OMV-mediated tolerance of P. gingivalis is characterized by selective abrogation of tumor necrosis factor (TNF). Neutralization of interleukin-10 (IL-10) during OMV challenge partially restores monocyte responsiveness toP. gingivalis; full reactivity toP. gingivalis can be restored by inhibition of mTOR signaling, which we previously identified as the major signaling pathway promoting Toll-like receptor 2 and Toll-like receptor 4 (TLR2/4)-mediated tolerance in monocytes. However, despite previous reports emphasizing a central role of TLR2 in innate immune recognition of P. gingivalis, our current findings highlight a selective role of TLR4 in the promotion of OMV-mediated TNF tolerance: only blockade of TLR4-and not of TLR2-restores responsiveness toP. gingivalis Of further note, OMV-mediated tolerance is preserved in the presence of cytochalasin B and chloroquine, indicating that triggering of surface TLR4 is sufficient for this effect. Taking the results together, we propose that P. gingivalis OMV contribute to local immune evasion of P. gingivalis by hampering the host response.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
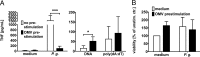


References
-
- Shah HN, Gharbia SE. 1995. The biochemical milieu of the host in the selection of anaerobic species in the oral cavity. Clin Infect Dis 20(Suppl 2):S291–S300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

