Structural studies of the C-terminal tail of polycystin-2 (PC2) reveal insights into the mechanisms used for the functional regulation of PC2
- PMID: 26857659
- PMCID: PMC4967733
- DOI: 10.1113/JP270933
Structural studies of the C-terminal tail of polycystin-2 (PC2) reveal insights into the mechanisms used for the functional regulation of PC2
Abstract
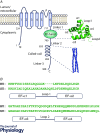
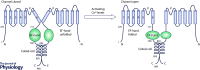
Mutations in polycystin-2 (PC2) lead to autosomal dominant polycystic kidney disease (ADPKD). The molecular mechanism linking mutations in PC2 and the pathogenesis of ADPKD is not well understood. Therefore, understanding the functional regulation of PC2 and its interaction with other proteins under both physiological and pathogenic conditions is important for elucidating the disease mechanism and identifying potential molecular targets for treatment. Normally, PC2 functions as a calcium-permeable channel whose activity is regulated by calcium binding to the C-terminal domain of PC2 (PC2 Cterm). The PC2 Cterm is also involved in the PC2 channel assembly and hetero-oligomerization with other binding partners in cells. Different functional domains of the PC2 Cterm have been studied using structural approaches. Within the PC2 Cterm, there is a calcium-binding EF-hand domain, crucial for the calcium-dependent activity of the PC2 channel. Downstream of the EF-hand domain lies a coiled-coil region, which is involved in the assembly and hetero-interaction of the PC2 protein. The PC2 Cterm can form an oligomer, mediated by the coiled-coil region. Although PC2 Cterm has been extensively studied for its relationship with ADPKD and its importance in PC2 regulation, there are misunderstandings with respect to the definition of the domain topology within the PC2 Cterm and the functional role of each domain. Here, we review previous studies that connect the molecular properties of the domains of PC2 Cterm to distinct aspects of PC2 functions and regulation.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures

Similar articles
-
Conformational dynamics of Ca2+-dependent responses in the polycystin-2 C-terminal tail.Biochem J. 2016 Feb 1;473(3):285-96. doi: 10.1042/BJ20151031. Epub 2015 Nov 16. Biochem J. 2016. PMID: 26574436 Free PMC article.
-
Oligomerization of the polycystin-2 C-terminal tail and effects on its Ca2+-binding properties.J Biol Chem. 2015 Apr 17;290(16):10544-54. doi: 10.1074/jbc.M115.641803. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716316 Free PMC article.
-
Structure of the EF-hand domain of polycystin-2 suggests a mechanism for Ca2+-dependent regulation of polycystin-2 channel activity.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9176-81. doi: 10.1073/pnas.0912295107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439752 Free PMC article.
-
New emerging roles of Polycystin-2 in the regulation of autophagy.Int Rev Cell Mol Biol. 2020;354:165-186. doi: 10.1016/bs.ircmb.2020.02.006. Epub 2020 Mar 11. Int Rev Cell Mol Biol. 2020. PMID: 32475472 Review.
-
Structure and function of polycystins: insights into polycystic kidney disease.Nat Rev Nephrol. 2019 Jul;15(7):412-422. doi: 10.1038/s41581-019-0143-6. Nat Rev Nephrol. 2019. PMID: 30948841 Review.
Cited by
-
PKD2/polycystin-2 induces autophagy by forming a complex with BECN1.Autophagy. 2021 Jul;17(7):1714-1728. doi: 10.1080/15548627.2020.1782035. Epub 2020 Jun 30. Autophagy. 2021. PMID: 32543276 Free PMC article.
-
Non-selective cationic channels in chemical and physical stress.J Physiol. 2016 Aug 1;594(15):4139. doi: 10.1113/JP270937. J Physiol. 2016. PMID: 27477608 Free PMC article. No abstract available.
-
Ca2+ Regulation of TRP Ion Channels.Int J Mol Sci. 2018 Apr 23;19(4):1256. doi: 10.3390/ijms19041256. Int J Mol Sci. 2018. PMID: 29690581 Free PMC article. Review.
-
Clinical and genetic characteristics of Korean autosomal dominant polycystic kidney disease patients.Korean J Intern Med. 2021 Jul;36(4):767-779. doi: 10.3904/kjim.2021.176. Epub 2021 Jul 1. Korean J Intern Med. 2021. PMID: 34237823 Free PMC article. Review.
-
Natural-derived compounds and their mechanisms in potential autosomal dominant polycystic kidney disease (ADPKD) treatment.Clin Exp Nephrol. 2021 Nov;25(11):1163-1172. doi: 10.1007/s10157-021-02111-x. Epub 2021 Jul 12. Clin Exp Nephrol. 2021. PMID: 34254206 Review.
References
-
- Anyatonwu GI & Ehrlich BE (2005). Organic cation permeation through the channel formed by polycystin‐2. J Biol Chem 280, 29488–29493. - PubMed
-
- Behn D, Bosk S, Hoffmeister H, Janshoff A, Witzgall R & Steinem C (2010). Quantifying the interaction of the C‐terminal regions of polycystin‐2 and polycystin‐1 attached to a lipid bilayer by means of QCM. Biophys Chem 150, 47–53. - PubMed
-
- Cai Y, Anyatonwu G, Okuhara D, Lee KB, Yu Z, Onoe T, Mei CL, Qian Q, Geng L, Wiztgall R, Ehrlich BE & Somlo S (2004). Calcium dependence of polycystin‐2 channel activity is modulated by phosphorylation at Ser812 . J Biol Chem 279, 19987–19995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

