First clinical study of a novel complete metal-free ceramic total knee replacement system
- PMID: 26857704
- PMCID: PMC4745159
- DOI: 10.1186/s13018-016-0352-7
First clinical study of a novel complete metal-free ceramic total knee replacement system
Abstract
Background: The aim of the study was to evaluate the safety and efficacy of a novel metal-free ceramic total knee replacement system.
Methods: Thirty-eight primary total knee arthroplasties (TKAs) were performed on 34 patients using the metal-free BPK-S ceramic total knee replacement system with both the femoral and tibial components of an alumina/zirconia ceramic composite. The clinical outcome was evaluated pre- and postoperatively at 3 (n = 32 TKA) and 12 months (n = 32 TKA) using the Knee Society Score (KSS), the Oxford Knee Score and the EQ-5D. Safety analysis was performed by radiological examination and assessment of adverse events.
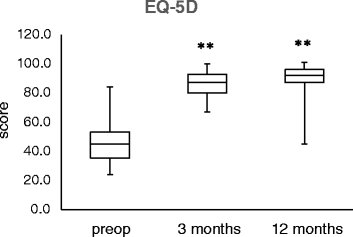
Results: Postoperatively, the KSS, Oxford Knee Score and EQ-5D improved significantly at 3 and 12 months (p < 0.001). Non-progressive partial radiolucent lines were observed in six cases, but there was no osteolysis and no implant loosening. Induction or exacerbation of allergies did not occur during the follow-up.
Conclusions: The metal-free BPK-S ceramic total knee replacement system proved to be a safe and clinically efficient alternative to metal implants in this short-term follow-up study.
Figures





Similar articles
-
Metal hypersensitivity after knee arthroplasty: fact or fiction?Acta Biomed. 2017 Jun 7;88(2S):78-83. doi: 10.23750/abm.v88i2-S.6517. Acta Biomed. 2017. PMID: 28657568 Free PMC article. Review.
-
Long term follow-up of a completely metal free total knee endoprosthesis in comparison to an identical metal counterpart.Sci Rep. 2024 Sep 9;14(1):20958. doi: 10.1038/s41598-024-71256-y. Sci Rep. 2024. PMID: 39251687 Free PMC article.
-
Prospective Comparison of a Metal-Free Ceramic Total Knee Arthroplasty with an Identical Metal System.Z Orthop Unfall. 2018 Feb;156(1):46-52. doi: 10.1055/s-0043-118600. Epub 2018 Feb 22. Z Orthop Unfall. 2018. PMID: 29471557 Clinical Trial. English.
-
Prospective Mid-Term Results of a Completely Metal-Free Ceramic Total Knee Endoprosthesis: A Concise Follow-Up of a Previous Report.J Arthroplasty. 2021 Sep;36(9):3161-3167. doi: 10.1016/j.arth.2021.05.007. Epub 2021 May 11. J Arthroplasty. 2021. PMID: 34090690
-
Clinical outcomes of ceramic femoral prosthesis in total knee arthroplasty: a systematic review.J Orthop Surg Res. 2019 Feb 19;14(1):57. doi: 10.1186/s13018-019-1090-4. J Orthop Surg Res. 2019. PMID: 30782186 Free PMC article.
Cited by
-
Effects of perceptions of care, medical advice, and hospital quality on patient satisfaction after primary total knee replacement: A cross-sectional study.PLoS One. 2017 Jun 13;12(6):e0178591. doi: 10.1371/journal.pone.0178591. eCollection 2017. PLoS One. 2017. PMID: 28609474 Free PMC article.
-
Metal hypersensitivity after knee arthroplasty: fact or fiction?Acta Biomed. 2017 Jun 7;88(2S):78-83. doi: 10.23750/abm.v88i2-S.6517. Acta Biomed. 2017. PMID: 28657568 Free PMC article. Review.
-
Femtosecond Laser Irradiation to Zirconia Prior to Calcium Phosphate Coating Enhances Osteointegration of Zirconia in Rabbits.J Funct Biomater. 2024 Feb 11;15(2):42. doi: 10.3390/jfb15020042. J Funct Biomater. 2024. PMID: 38391895 Free PMC article.
-
Ceramic Total Knee Arthroplasty: Ready to Go?Joints. 2017 Oct 26;5(4):224-228. doi: 10.1055/s-0037-1607428. eCollection 2017 Dec. Joints. 2017. PMID: 29270560 Free PMC article. Review.
-
Advancements in Biomedical Sensors for Early Detection of Failure in Hip and Knee Implants: Scoping Review on Potential Sensors for Implant Integration.Ann Biomed Eng. 2025 Jul 2. doi: 10.1007/s10439-025-03780-5. Online ahead of print. Ann Biomed Eng. 2025. PMID: 40603592 Review.
References
-
- Naudie DD, Ammeen DJ, Engh GA, Rorabeck CH. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg. 2007;15(1):53–64. - PubMed
-
- Yasuda K, Miyagi N, Kaneda K. Low friction total knee arthroplasty with the alumina ceramic condylar prosthesis. Bull Hosp Jt Dis. 1993;53(2):15–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

