Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes
- PMID: 26857840
- PMCID: PMC4746733
- DOI: 10.1038/srep20764
Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes
Abstract
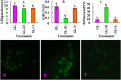
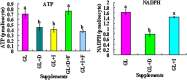
Although there are many reports on the effect of glucose metabolism on oocyte nuclear maturation, there are few studies on its effect on ooplasmic maturation. By manipulating glucose metabolism pathways using a maturation medium that could support oocyte nuclear maturation but only a limited blastocyst formation without glucose, this study determined effects of glucose metabolism pathways on ooplasmic maturation. During maturation of cumulus-oocyte-complexes (COCs) with glucose, the presence of PPP inhibitor, DHEA or glycolysis inhibitor, iodoacetate significantly decreased blastocyst rates, intraoocyte glutathione and ATP. While blastocyst rates, GSH/GSSG ratio and NADPH were higher, ROS was lower significantly in COCs matured with iodoacetate than with DHEA. Fructose-6-phosphate overcame the inhibitory effect of DHEA on PPP. During maturation of COCs with pyruvate, electron transport inhibitor, rotenone or monocarboxylate transfer inhibitor, 4-CIN significantly decreased blastocyst rates. Cumulus-denuded oocytes had a limited capacity to use glucose or lactate, but they could use pyruvate to support maturation. In conclusion, whereas glycolysis promoted ooplasmic maturation mainly by supplying energy, PPP facilitated ooplasmic maturation to a greater extent by both reducing oxidative stress and supplying energy through providing fructose-6-phosphate for glycolysis. Pyruvate was transferred by monocarboxylate transporters and utilized through mitochondrial electron transport to sustain ooplasmic maturation.
Figures


References
-
- Sirard M. A., Richard F., Blondin P. & Robert C. Contribution of the oocyte to embryo quality. Theriogenology 65, 126–136 (2006). - PubMed
-
- Eppig J. J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Devel 8, 485–489 (1996). - PubMed
-
- Ferreira E. M. et al. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71, 836–848 (2009). - PubMed
-
- Sorensen R. A. & Wassarman P. M. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol 50, 531–536 (1976). - PubMed
-
- Eppig J. J. & Schroeder A. C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 41, 268–276 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

