A randomized phase 3 study on the optimization of the combination of bevacizumab with FOLFOX/OXXEL in the treatment of patients with metastatic colorectal cancer-OBELICS (Optimization of BEvacizumab scheduLIng within Chemotherapy Scheme)
- PMID: 26857924
- PMCID: PMC4746902
- DOI: 10.1186/s12885-016-2102-y
A randomized phase 3 study on the optimization of the combination of bevacizumab with FOLFOX/OXXEL in the treatment of patients with metastatic colorectal cancer-OBELICS (Optimization of BEvacizumab scheduLIng within Chemotherapy Scheme)
Abstract
Background: Despite the improvements in diagnosis and treatment, colorectal cancer (CRC) is the second cause of cancer deaths in both sexes. Therefore, research in this field remains of great interest. The approval of bevacizumab, a humanized anti-vascular endothelial growth factor (VEGF) monoclonal antibody, in combination with a fluoropyrimidine-based chemotherapy in the treatment of metastatic CRC has changed the oncology practice in this disease. However, the efficacy of bevacizumab-based treatment, has thus far been rather modest. Efforts are ongoing to understand the better way to combine bevacizumab and chemotherapy, and to identify valid predictive biomarkers of benefit to avoid unnecessary and costly therapy to nonresponder patients. The BRANCH study in high-risk locally advanced rectal cancer patients showed that varying bevacizumab schedule may impact on the feasibility and efficacy of chemo-radiotherapy.
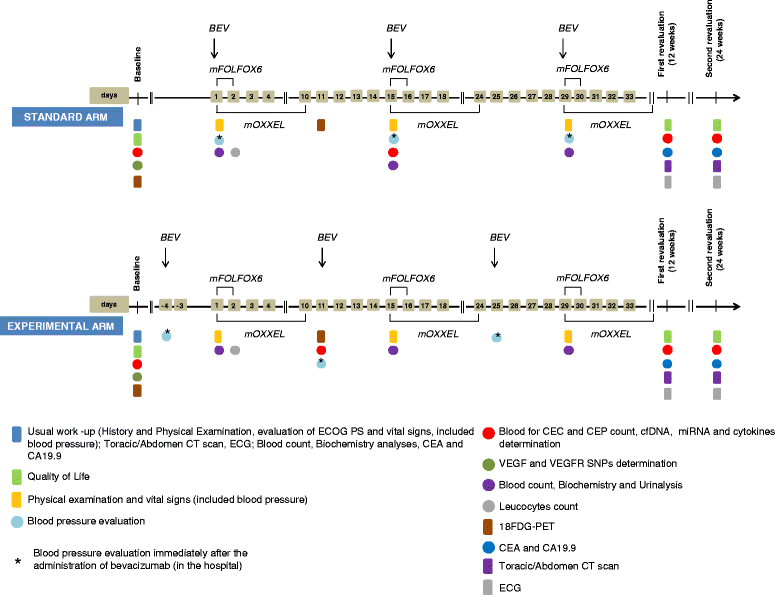
Methods/design: OBELICS is a multicentre, open-label, randomised phase 3 trial comparing in mCRC patients two treatment arms (1:1): standard concomitant administration of bevacizumab with chemotherapy (mFOLFOX/OXXEL regimen) vs experimental sequential bevacizumab given 4 days before chemotherapy, as first or second treatment line. Primary end point is the objective response rate (ORR) measured according to RECIST criteria. A sample size of 230 patients was calculated allowing reliable assessment in all plausible first-second line case-mix conditions, with a 80% statistical power and 2-sided alpha error of 0.05. Secondary endpoints are progression free-survival (PFS), overall survival (OS), toxicity and quality of life. The evaluation of the potential predictive role of several circulating biomarkers (circulating endothelial cells and progenitors, VEGF and VEGF-R SNPs, cytokines, microRNAs, free circulating DNA) as well as the value of the early [(18)F]-Fluorodeoxyglucose positron emission tomography (FDG-PET) response, are the objectives of the traslational project.
Discussion: Overall this study could optimize bevacizumab scheduling in combination with chemotherapy in mCRC patients. Moreover, correlative studies could improve the knowledge of the mechanisms by which bevacizumab enhance chemotherapy effect and could identify early predictors of response. EudraCT Number: 2011-004997-27 TRIAL REGISTRATION: ClinicalTrials.gove number, NCT01718873.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical