Aflibercept for neovascular age-related macular degeneration
- PMID: 26857947
- PMCID: PMC5030844
- DOI: 10.1002/14651858.CD011346.pub2
Aflibercept for neovascular age-related macular degeneration
Abstract
Background: Central vision loss caused by age-related macular degeneration (AMD) is the leading cause of blindness among the elderly in developed countries. Neovascular AMD is characterized by choroidal neovascularization (CNV). Growth of new blood vessels in patients with neovascular AMD is driven by a complex process that involves a signal protein called vascular endothelial growth factor A (VEGF-A). Anti-VEGF drugs that block this protein include ranibizumab, bevacizumab, and aflibercept.
Objectives: To assess and compare the effectiveness and safety of intravitreal injections of aflibercept versus ranibizumab, bevacizumab, or sham for treatment of patients with neovascular AMD.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (Issue 11, 2015), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to November 2015), EMBASE (January 1980 to November 2015), PubMed (1948 to November 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to November 2015), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com) (last searched December 4, 2014), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic search for trials. We last searched the electronic databases on November 30, 2015.
Selection criteria: We included randomized controlled trials (RCTs) in which aflibercept monotherapy was compared with ranibizumab, bevacizumab, or sham for participants with neovascular AMD who were treatment-naive.
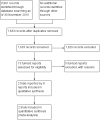
Data collection and analysis: We used standard methodological procedures of The Cochrane Collaboration for screening, data abstraction, and study assessment. Two review authors independently screened records, abstracted data, and assessed risk of bias of included studies; we resolved discrepancies by discussion or with the help of a third review author when needed.
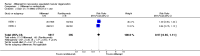
Main results: We included two RCTs (total of 2457 participants, 2457 eyes). Trial participants had neovascular AMD with active subfoveal choroidal neovascular lesions. Both trials followed the same protocol and compared aflibercept at various doses versus ranibizumab, but they were carried out in different countries. One trial enrolled participants from the United States and Canada, and the second trial was conducted at 172 sites in Europe, Asia Pacific, Latin America, and the Middle East. The overall quality of the evidence was high, and included trials were at low risk for most bias domains assessed; however, both trials were funded by the manufacturers of aflibercept. For the purposes of analysis, we combined aflibercept groups regardless of dosing and analyzed them as a single group.Visual acuity outcomes were similar between aflibercept and ranibizumab groups; at one year, participants in the aflibercept groups showed mean change in best-corrected visual acuity (BCVA) from baseline similar to that of participants in the ranibizumab groups (mean difference (MD) -0.15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, 95% confidence interval (95% CI) -1.47 to 1.17; high-quality evidence). At two years, the mean change in BCVA from baseline was 7.2 ETDRS letters for aflibercept groups versus 7.9 for ranibizumab groups. Sufficient data were not available for calculation of confidence intervals.The proportion of participants who gained 15 or more letters of BCVA by one year of follow-up was approximately 32% for both aflibercept and ranibizumab (RR 0.97, 95% CI 0.85 to 1.11; high-quality evidence), and by two years of follow-up was approximately 31% (RR 0.98, 95% CI 0.85 to 1.12; high-quality evidence). Similar small proportions of participants in the aflibercept and ranibizumab groups lost 15 or more letters of BCVA at one year (RR 0.89, 95% CI 0.61 to 1.30; high-quality evidence); this outcome was not reported for two-year follow-up. Data were not reported on the proportion of participants with BCVA worse than 20/200 at one- or two-year follow-up.Participants treated with aflibercept or ranibizumab showed similar improvement in morphological outcomes, as assessed from images (central retinal thickness and CNV size). At one year, the proportion of eyes that achieved dry retina was similar between aflibercept and ranibizumab groups (absence of cystic intraretinal fluid and subretinal fluid on optical coherence tomography (OCT); RR 1.06, 95% CI 0.98 to 1.14; high-quality evidence). In addition, investigators reported no difference in reduction of CNV area between aflibercept- and ranibizumab-treated eyes at one year (MD -0.24 mm(2), 95% CI -0.78 to 0.29; high-quality evidence). Data were not reported for the proportion of eyes with absence of leakage on fluorescein angiography at one- or two-year follow-up.Overall, occurrence of serious systemic adverse events was similar and comparable in aflibercept- and ranibizumab-treated groups at one year (RR 0.99, 95% CI 0.79 to 1.25). Risk of any serious ocular adverse event was lower in the aflibercept group than in the ranibizumab group, but the risk estimate is imprecise (RR 0.62, 95% CI 0.36 to 1.07). As the result of imprecision, we graded the quality of evidence for all adverse events as moderate.
Authors' conclusions: Results of this review document the comparative effectiveness of aflibercept versus ranibizumab for visual acuity and morphological outcomes in eyes with neovascular AMD. Current available information on adverse effects of each medication suggests that the safety profile of aflibercept is comparable with that of ranibizumab; however, the number of participants who experienced adverse events was small, leading to imprecise estimates of absolute and relative effect sizes. The eight-week dosing regimen of aflibercept represents reduced treatment requirements in comparison with monthly dosing regimens and thus has the potential to reduce treatment burden and risks associated with frequent injections.
Conflict of interest statement
Dr. Salman Sarwar: none known. Ms. Elizabeth Clearfield: none known. Dr. Mohamed K Soliman: none known. Dr. Mohammad A Sadiq: none known. Dr. Andrew J Baldwin: none known. Dr. Mostafa Hanout: none known. Dr. Aniruddha Agarwal: none known. Dr. Yasir J Sepah: none known. Dr. Diana V. Do's institution has received research funding from Genentech and Regeneron. Dr. Do has received consulting fees or honoraria from Allergan, Bayer, Genentech, Oligasis, and Regeneron, and has served as a consultant to Genentech and Regeneron within the past three years. Dr. Do chairs the Steering Committee for the VISTA/VIVID Study. Dr. Quan Dong Nguyen's institution has received research funding from Genentech, Regeneron, AbbVie, Psivida, and XOMA. Dr. Nguyen has received consulting fees or honoraria from Allergan, Bausch and Lomb, Bayer, Genentech, Oligasis, Regeneron, and Santen. He has served on the Scientific Advisory Board for AbbVie, Genentech, Regeneron, Santen, and XOMA within the past three years. Dr. Nguyen chairs the Steering Committee for the RISE/RIDE Study, as well as the EYEGUARD, SAKURA, and VISUAL Studies.
Figures


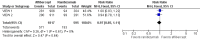

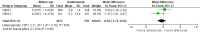








Update of
References
References to studies included in this review
VIEW 1 {published data only}
-
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age‐related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802‐10. - PubMed
-
- Heier JS, Brown DM, Chong V, Korobelnik J‐F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap‐Eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. - PubMed
-
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age‐related macular degeneration: 52‐week subgroup analyses from the combined VIEW studies. Clinical and Experimental Ophthalmology 2012;40(Suppl 1):41‐56.
-
- Richard G, Monés J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology 2015;122(12):2497‐503. - PubMed
-
- Schmidt‐Erfurth U, Kaiser PK, Korobelnik J‐F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age‐related macular degeneration. Ninety‐six week results of the VIEW studies. Ophthalmology 2014;121(1):193‐201. - PubMed
VIEW 2 {published data only}
-
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age‐related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802‐10. - PubMed
-
- Heier JS, Brown DM, Chong V, Korobelnik J‐F, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF Trap‐Eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. - PubMed
-
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age‐related macular degeneration: 52‐week subgroup analyses from the combined VIEW studies. Clinical and Experimental Ophthalmology 2012;40(Suppl 1):41‐56.
-
- Richard G, Monés J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology 2015;122(12):2497‐503. - PubMed
References to studies excluded from this review
CLEAR‐AMD 1 {published data only}
-
- Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, et al. A phase I trial of an IV‐administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age‐related macular degeneration. Ophthalmology 2006;113(9):1522.e1‐14. - PubMed
Elshout 2014 {published data only}
-
- Elshout M, Reis MI, Webers CA, Schouten JS. The cost‐utility of aflibercept for the treatment of age‐related macular degeneration compared to bevacizumab and ranibizumab and the influence of model parameters. Graefe's Archive for Clinical and Experimental Ophthalmology 2014;252(12):1911‐20. - PubMed
Yoshida 2014 {published data only}
-
- Yoshida I, Shiba T, Taniquchi H, Takahashi M, Murano T, Hiruta N, et al. Evaluation of plasma vascular endothelial growth factor levels after intravitreal injection of ranibizumab and aflibercept for exudative age‐related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 2014;252(9):1483‐9. - PubMed
Zehetner 2015 {published data only}
-
- Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, et al. Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age‐related macular degeneration: a randomised, prospective trial. Acta Ophthalmologica 2015;93(2):e154‐9. - PubMed
Zinkernagel 2015 {published data only}
-
- Zinkernagel MS, Schorno P, Ebneter A, Wolf S. Scleral thinning after repeated intravitreal injections of antivascular endothelial growth factor agents in the same quadrant. Investigative Ophthalmology & Visual Science 2015;56(3):1894‐900. - PubMed
Additional references
AAO 2015
-
- American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern® Guidelines. Age‐Related Macular Degeneration. San Francisco, CA: American Academy of Ophthalmology; 2015. www.aao.org/ppp.
AREDS 2005
Bird 1995
-
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age‐related maculopathy and age‐related macular degeneration. The International ARM Epidemiological Study Group. Survey of Ophthalmology 1995;39(5):367‐74. - PubMed
Brown 2006
-
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1432‐44. - PubMed
Brown 2011
-
- Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap‐eye in wet age‐related macular degeneration. Ophthalmology 2011;118(6):1089‐97. - PubMed
CATT Research Group 2011
Chakravarthy 2010
Childs 2004
Clemons 2003
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Do 2012
-
- Do DV, Gower EW, Cassard SD, Boyer D, Bressler NM, Bressler SB, et al. Detection of new‐onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology 2012;119(4):771‐8. - PubMed
Dong 2004
-
- Dong LM, Childs AL, Mangione CM, Bass EB, Bressler NM, Hawkins BS, et al. Health‐ and vision‐related quality of life among patients with choroidal neovascularization secondary to age‐related macular degeneration at enrollment in randomized trials of submacular surgery: SST Report No. 4. American Journal of Ophthalmology 2004;138(1):91‐108. - PubMed
FDA 2011
-
- US Food, Drug Administration. FDA news release (2011): FDA approves Eylea for eye disorder in older people. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280601.htm (accessed 16 December 2013).
Ferrara 2009
-
- Ferrara N. VEGF‐A: a critical regulator of blood vessel growth. European Cytokine Network 2009;20(4):158‐63. - PubMed
Fine 1986
-
- Fine AM, Elman MJ, Ebert JE, Prestia PA, Starr JS, Fine SL. Earliest symptoms caused by neovascular membranes in the macula. Archives of Ophthalmology 1986;104(4):513‐4. - PubMed
Friedman 2004
-
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age‐related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564‐72. - PubMed
Haller 2013
-
- Haller JA. Current anti‐vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology 2013;120(5 Suppl):S3‐7. - PubMed
Heier 2011
-
- Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1‐year results of CLEAR‐IT 2, a phase 2 study of vascular endothelial growth factor trap‐eye dosed as‐needed after 12‐week fixed dosing. Ophthalmology 2011;118(6):1098‐106. - PubMed
Heier 2012
-
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap‐eye) in wet age‐related macular degeneration. Ophthalmology 2012;119(12):2537‐48. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holash 2002
Hyman 2002
-
- Hyman L, Neborsky R. Risk factors for age‐related macular degeneration: an update. Current Opinion in Ophthalmology 2002;13(3):171‐5. - PubMed
IVAN Trial 2013
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age‐related choroidal neovascularisation: 2‐year findings of the IVAN randomised controlled trial. Lancet 2013;382(9900):1258‐67. - PubMed
Jansen 2013
-
- Jansen RM. The off‐label use of medication: the latest on the Avastin ‐ Lucentis debacle. Medicine and Law 2013;32(1):65‐77. - PubMed
Mangione 1999
-
- Mangione CM, Gutierrez PR, Lowe G, Orav EJ, Seddon JM. Influence of age‐related maculopathy on visual functioning and health‐related quality of life. American Journal of Ophthalmology 1999;128(1):45‐53. - PubMed
Miskala 2004
Mitchell 2011
-
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti‐VEGF agents used for treating neovascular age‐related macular degeneration: comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion 2011;27(7):1465‐75. - PubMed
Moja 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenfeld 2006
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1419‐31. - PubMed
Rudge 2007
-
- Rudge JS, Holash J, Hylton D, Russell M, Jiang S, Leidich R, et al. VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proceedings of the National Academy of Sciences of the United States of America 2007;104(47):18363‐70. - PMC - PubMed
Schmid 2015
-
- Schmid MK, Bachmann LM, Fäs L, Kessels AG, Job OM, Thiel MA. Efficacy and adverse events of aflibercept, ranibizumab and bevacizumab in age‐related macular degeneration: a trade‐off analysis. British Journal of Ophthalmology 2015;99(2):141‐6. - PubMed
Schmucker 2010
-
- Schmucker C, Ehlken C, Hansen LL, Antes G, Agostini HT, Lelgemann M. Intravitreal bevacizumab (Avastin) vs. ranibizumab (Lucentis) for the treatment of age‐related macular degeneration: a systematic review. Current Opinion in Ophthalmology 2010;21(3):218‐26. - PubMed
Schmucker 2012
Schultz 2003
-
- Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, et al. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN‐1 is associated with age‐related macular degeneration in a large family. Human Molecular Genetics 2003;12(24):3315‐23. - PubMed
Solomon 2014
Stewart 2008
-
- Stewart MW, Rosenfeld PJ. Predicted biological activity of intravitreal VEGF Trap. British Journal of Ophthalmology 2008;92(5):667‐8. - PubMed
Thomas 2013
Thornton 2005
-
- Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age‐related macular degeneration: a review of association. Eye 2005;19(9):935‐44. - PubMed
Wong 2008
-
- Wong TY, Chakravarthy U, Kelin R, Mitchell P, Zlateva G, Buggage R, et al. The natural history and prognosis of neovascular age‐related macular degeneration: a systematic review of the literature and meta‐analysis. Ophthalmology 2008;115(1):116‐26. - PubMed
Wong 2014
-
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng CY, et al. Global prevalence of age‐related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta‐analysis. The Lancet Global Health 2014;2(2):e106‐16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

