WrpA Is an Atypical Flavodoxin Family Protein under Regulatory Control of the Brucella abortus General Stress Response System
- PMID: 26858101
- PMCID: PMC4859584
- DOI: 10.1128/JB.00982-15
WrpA Is an Atypical Flavodoxin Family Protein under Regulatory Control of the Brucella abortus General Stress Response System
Abstract
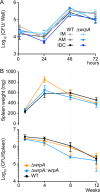
The general stress response (GSR) system of the intracellular pathogen Brucella abortus controls the transcription of approximately 100 genes in response to a range of stress cues. The core genetic regulatory components of the GSR are required for B. abortus survival under nonoptimal growth conditions in vitro and for maintenance of chronic infection in an in vivo mouse model. The functions of the majority of the genes in the GSR transcriptional regulon remain undefined. bab1_1070 is among the most highly regulated genes in this regulon: its transcription is activated 20- to 30-fold by the GSR system under oxidative conditions in vitro. We have solved crystal structures of Bab1_1070 and demonstrate that it forms a homotetrameric complex that resembles those of WrbA-type NADH:quinone oxidoreductases, which are members of the flavodoxin protein family. However, B. abortus WrbA-related protein (WrpA) does not bind flavin cofactors with a high affinity and does not function as an NADH:quinone oxidoreductase in vitro. Soaking crystals with flavin mononucleotide (FMN) revealed a likely low-affinity binding site adjacent to the canonical WrbA flavin binding site. Deletion of wrpA (ΔwrpA) does not compromise cell survival under acute oxidative stress in vitro or attenuate infection in cell-based or mouse models. However, a ΔwrpA strain does elicit increased splenomegaly in a mouse model, suggesting that WrpA modulates B. abortus interaction with its mammalian host. Despite high structural homology with canonical WrbA proteins, we propose that B. abortus WrpA represents a functionally distinct member of the diverse flavodoxin family.
Importance: Brucella abortus is an etiological agent of brucellosis, which is among the most common zoonotic diseases worldwide. The general stress response (GSR) regulatory system of B. abortus controls the transcription of approximately 100 genes and is required for maintenance of chronic infection in a murine model; the majority of GSR-regulated genes remain uncharacterized. We present in vitro and in vivo functional and structural analyses of WrpA, whose expression is strongly induced by GSR under oxidative conditions. Though WrpA is structurally related to NADH:quinone oxidoreductases, it does not bind redox cofactors in solution, nor does it exhibit oxidoreductase activity in vitro. However, WrpA does affect spleen inflammation in a murine infection model. Our data provide evidence that WrpA forms a new functional class of WrbA/flavodoxin family proteins.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
The Brucella abortus general stress response system regulates chronic mammalian infection and is controlled by phosphorylation and proteolysis.J Biol Chem. 2013 May 10;288(19):13906-16. doi: 10.1074/jbc.M113.459305. Epub 2013 Apr 1. J Biol Chem. 2013. PMID: 23546883 Free PMC article.
-
Conserved ABC Transport System Regulated by the General Stress Response Pathways of Alpha- and Gammaproteobacteria.J Bacteriol. 2017 Feb 14;199(5):e00746-16. doi: 10.1128/JB.00746-16. Print 2017 Mar 1. J Bacteriol. 2017. PMID: 27994018 Free PMC article.
-
Characterization of Three Small Proteins in Brucella abortus Linked to Fucose Utilization.J Bacteriol. 2018 Aug 24;200(18):e00127-18. doi: 10.1128/JB.00127-18. Print 2018 Sep 15. J Bacteriol. 2018. PMID: 29967118 Free PMC article.
-
The Brucella abortus virulence regulator, LovhK, is a sensor kinase in the general stress response signalling pathway.Mol Microbiol. 2014 Nov;94(4):913-25. doi: 10.1111/mmi.12809. Epub 2014 Oct 19. Mol Microbiol. 2014. PMID: 25257300 Free PMC article.
-
Learning from the master: targets and functions of the CtrA response regulator in Brucella abortus and other alpha-proteobacteria.FEMS Microbiol Rev. 2018 Jul 1;42(4):500-513. doi: 10.1093/femsre/fuy019. FEMS Microbiol Rev. 2018. PMID: 29733367 Review.
Cited by
-
Brucella abortus ΔrpoE1 confers protective immunity against wild type challenge in a mouse model of brucellosis.Vaccine. 2016 Sep 30;34(42):5073-5081. doi: 10.1016/j.vaccine.2016.08.076. Epub 2016 Aug 31. Vaccine. 2016. PMID: 27591954 Free PMC article.
-
Mining the Flavoproteome of Brucella ovis, the Brucellosis Causing Agent in Ovis aries.Microbiol Spectr. 2022 Apr 27;10(2):e0229421. doi: 10.1128/spectrum.02294-21. Epub 2022 Mar 22. Microbiol Spectr. 2022. PMID: 35315701 Free PMC article.
-
An Assay to Determine NAD(P)H: Quinone Oxidoreductase Activity in Cell Extracts from Candida glabrata.Bio Protoc. 2021 Nov 5;11(21):e4210. doi: 10.21769/BioProtoc.4210. eCollection 2021 Nov 5. Bio Protoc. 2021. PMID: 34859125 Free PMC article.
-
Molecular control of gene expression by Brucella BaaR, an IclR-type transcriptional repressor.J Biol Chem. 2018 May 11;293(19):7437-7456. doi: 10.1074/jbc.RA118.002045. Epub 2018 Mar 22. J Biol Chem. 2018. PMID: 29567835 Free PMC article.
-
Intermolecular correlations are necessary to explain diffuse scattering from protein crystals.IUCrJ. 2018 Feb 21;5(Pt 2):211-222. doi: 10.1107/S2052252518001124. eCollection 2018 Mar 1. IUCrJ. 2018. PMID: 29765611 Free PMC article.
References
-
- Mishra A, Mishra KP. 2015. Bacterial resistance mechanism against oxidative stress. J Med Pharm Innov 2:1–9.
-
- Poole K. 2008. Bacterial multidrug efflux pumps serve other functions. Microbe 3:179–185.
-
- Cabiscol E, Tamarit J, Ros J. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

