Molecular Characterization of the Vacuolating Autotransporter Toxin in Uropathogenic Escherichia coli
- PMID: 26858103
- PMCID: PMC4859599
- DOI: 10.1128/JB.00791-15
Molecular Characterization of the Vacuolating Autotransporter Toxin in Uropathogenic Escherichia coli
Abstract
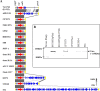
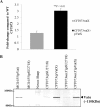
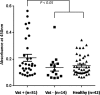
The vacuolating autotransporter toxin (Vat) contributes to uropathogenic Escherichia coli (UPEC) fitness during systemic infection. Here, we characterized Vat and investigated its regulation in UPEC. We assessed the prevalence of vat in a collection of 45 UPEC urosepsis strains and showed that it was present in 31 (68%) of the isolates. The isolates containing the vat gene corresponded to three major E. coli sequence types (ST12, ST73, and ST95), and these strains secreted the Vat protein. Further analysis of the vat genomic locus identified a conserved gene located directly downstream of vat that encodes a putative MarR-like transcriptional regulator; we termed this gene vatX The vat-vatX genes were present in the UPEC reference strain CFT073, and reverse transcriptase PCR (RT-PCR) revealed that the two genes are cotranscribed. Overexpression of vatX in CFT073 led to a 3-fold increase in vat gene transcription. The vat promoter region contained three putative nucleation sites for the global transcriptional regulator histone-like nucleoid structuring protein (H-NS); thus, the hns gene was mutated in CFT073 (to generate CFT073 hns). Western blot analysis using a Vat-specific antibody revealed a significant increase in Vat expression in CFT073 hns compared to that in wild-type CFT073. Direct H-NS binding to the vat promoter region was demonstrated using purified H-NS in combination with electrophoresis mobility shift assays. Finally, Vat-specific antibodies were detected in plasma samples from urosepsis patients infected by vat-containing UPEC strains, demonstrating that Vat is expressed during infection. Overall, this study has demonstrated that Vat is a highly prevalent and tightly regulated immunogenic serine protease autotransporter protein of Enterobacteriaceae (SPATE) secreted by UPEC during infection.
Importance: Uropathogenic Escherichia coli (UPEC) is the major cause of hospital- and community-acquired urinary tract infections. The vacuolating autotransporter toxin (Vat) is a cytotoxin known to contribute to UPEC fitness during murine sepsis infection. In this study, Vat was found to be highly conserved and prevalent among a collection of urosepsis clinical isolates and was expressed at human core body temperature. Regulation of vat was demonstrated to be directly repressed by the global transcriptional regulator H-NS and upregulated by the downstream gene vatX (encoding a new MarR-type transcriptional regulator). Additionally, increased Vat-specific IgG titers were detected in plasma from corresponding urosepsis patients infected with vat-positive isolates. Hence, Vat is a highly conserved and tightly regulated urosepsis-associated virulence factor.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Identification and Characterization of a Phase-Variable Element That Regulates the Autotransporter UpaE in Uropathogenic Escherichia coli.mBio. 2018 Aug 7;9(4):e01360-18. doi: 10.1128/mBio.01360-18. mBio. 2018. PMID: 30087170 Free PMC article.
-
Molecular characterization of UpaB and UpaC, two new autotransporter proteins of uropathogenic Escherichia coli CFT073.Infect Immun. 2012 Jan;80(1):321-32. doi: 10.1128/IAI.05322-11. Epub 2011 Sep 19. Infect Immun. 2012. PMID: 21930758 Free PMC article.
-
The Vacuolating Autotransporter Toxin (Vat) of Escherichia coli Causes Cell Cytoskeleton Changes and Produces Non-lysosomal Vacuole Formation in Bladder Epithelial Cells.Front Cell Infect Microbiol. 2020 Jun 26;10:299. doi: 10.3389/fcimb.2020.00299. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670893 Free PMC article.
-
Presence of putative repeat-in-toxin gene tosA in Escherichia coli predicts successful colonization of the urinary tract.mBio. 2011 May 3;2(3):e00066-11. doi: 10.1128/mBio.00066-11. Print 2011. mBio. 2011. PMID: 21540363 Free PMC article.
-
Virulence and Fitness Determinants of Uropathogenic Escherichia coli.Microbiol Spectr. 2015 Aug;3(4):10.1128/microbiolspec.UTI-0015-2012. doi: 10.1128/microbiolspec.UTI-0015-2012. Microbiol Spectr. 2015. PMID: 26350328 Free PMC article. Review.
Cited by
-
Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection.Int J Mol Sci. 2023 Apr 11;24(8):7055. doi: 10.3390/ijms24087055. Int J Mol Sci. 2023. PMID: 37108218 Free PMC article. Review.
-
Comparative Pathogenomics of Escherichia coli: Polyvalent Vaccine Target Identification through Virulome Analysis.Infect Immun. 2021 Jul 15;89(8):e0011521. doi: 10.1128/IAI.00115-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 33941580 Free PMC article.
-
The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen.Microorganisms. 2023 Jan 30;11(2):344. doi: 10.3390/microorganisms11020344. Microorganisms. 2023. PMID: 36838308 Free PMC article. Review.
-
Clonal Diversity, Virulence Potential and Antimicrobial Resistance of Escherichia coli Causing Community Acquired Urinary Tract Infection in Switzerland.Front Microbiol. 2017 Dec 1;8:2334. doi: 10.3389/fmicb.2017.02334. eCollection 2017. Front Microbiol. 2017. PMID: 29250044 Free PMC article.
-
Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options.Int J Mol Sci. 2023 Jun 23;24(13):10537. doi: 10.3390/ijms241310537. Int J Mol Sci. 2023. PMID: 37445714 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

