Antithrombin III for critically ill patients
- PMID: 26858174
- PMCID: PMC6517014
- DOI: 10.1002/14651858.CD005370.pub3
Antithrombin III for critically ill patients
Abstract
Background: Critical illness is associated with uncontrolled inflammation and vascular damage which can result in multiple organ failure and death. Antithrombin III (AT III) is an anticoagulant with anti-inflammatory properties but the efficacy and any harmful effects of AT III supplementation in critically ill patients are unknown. This review was published in 2008 and updated in 2015.
Objectives: To examine:1. The effect of AT III on mortality in critically ill participants.2. The benefits and harms of AT III.We investigated complications specific and not specific to the trial intervention, bleeding events, the effect on sepsis and disseminated intravascular coagulation (DIC) and the length of stay in the intensive care unit (ICU) and in hospital in general.
Search methods: We searched the following databases from inception to 27 August 2015: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid SP), EMBASE (Ovid SP,), CAB, BIOSIS and CINAHL. We contacted the main authors of trials to ask for any missed, unreported or ongoing trials.
Selection criteria: We included randomized controlled trials (RCTs) irrespective of publication status, date of publication, blinding status, outcomes published, or language. We contacted the investigators and the trial authors in order to retrieve missing data. In this updated review we include trials only published as abstracts.
Data collection and analysis: Our primary outcome measure was mortality. Two authors each independently abstracted data and resolved any disagreements by discussion. We presented pooled estimates of the intervention effects on dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). We performed subgroup analyses to assess risk of bias, the effect of AT III in different populations (sepsis, trauma, obstetrics, and paediatrics), and the effect of AT III in patients with or without the use of concomitant heparin. We assessed the adequacy of the available number of participants and performed trial sequential analysis (TSA) to establish the implications for further research.
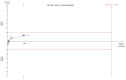
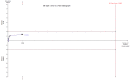
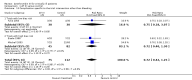
Main results: We included 30 RCTs with a total of 3933 participants (3882 in the primary outcome analyses).Combining all trials, regardless of bias, showed no statistically significant effect of AT III on mortality with a RR of 0.95 (95% CI 0.88 to 1.03), I² statistic = 0%, fixed-effect model, 29 trials, 3882 participants, moderate quality of evidence). For trials with low risk of bias the RR was 0.96 (95% Cl 0.88 to 1.04, I² statistic = 0%, fixed-effect model, 9 trials, 2915 participants) and for high risk of bias RR 0.94 (95% Cl 0.77 to 1.14, I² statistic = 0%, fixed-effect model, 20 trials, 967 participants).For participants with severe sepsis and DIC the RR for mortality was non-significant, 0.95 (95% Cl 0.88 to 1.03, I² statistic = 0%, fixed-effect model, 12 trials, 2858 participants, moderate quality of evidence).We conducted 14 subgroup and sensitivity analyses with respect to the different domains of risk of bias, but detected no statistically significant benefit in any subgroup analyses.Our secondary objective was to assess the benefits and harms of AT III. For complications specific to the trial intervention the RR was 1.26 (95% Cl 0.83 to 1.92, I² statistic = 0%, random-effect model, 3 trials, 2454 participants, very low quality of evidence). For complications not specific to the trial intervention, the RR was 0.71 (95% Cl 0.08 to 6.11, I² statistic = 28%, random-effects model, 2 trials, 65 participants, very low quality of evidence). For complications other than bleeding, the RR was 0.72 ( 95% Cl 0.42 to 1.25, I² statistic = 0%, fixed-effect model, 3 trials, 187 participants, very low quality of evidence). Eleven trials investigated bleeding events and we found a statistically significant increase, RR 1.58 (95% CI 1.35 to 1.84, I² statistic = 0%, fixed-effect model, 11 trials, 3019 participants, moderate quality of evidence) in the AT III group. The amount of red blood cells administered had a mean difference (MD) of 138.49 (95% Cl -391.35 to 668.34, I² statistic = 84%, random-effect model, 4 trials, 137 participants, very low quality of evidence). The effect of AT III in patients with multiple organ failure (MOF) was a MD of -1.24 (95% Cl -2.18 to -0.29, I² statistic = 48%, random-effects model, 3 trials, 156 participants, very low quality of evidence) and for patients with an Acute Physiology and Chronic Health Evaluation score (APACHE) at II and III the MD was -2.18 (95% Cl -4.36 to -0.00, I² statistic = 0%, fixed-effect model, 3 trials, 102 participants, very low quality of evidence). The incidence of respiratory failure had a RR of 0.93 (95% Cl 0.76 to 1.14, I² statistic = 32%, random-effects model, 6 trials, 2591 participants, moderate quality of evidence). AT III had no statistically significant impact on the duration of mechanical ventilation (MD 2.20 days, 95% Cl -1.21 to 5.60, I² statistic = 0%, fixed-effect model, 3 trials, 190 participants, very low quality of evidence); on the length of stay in the ICU (MD 0.24, 95% Cl -1.34 to 1.83, I² statistic = 0%, fixed-effect model, 7 trials, 376 participants, very low quality of evidence) or on the length of stay in hospital in general (MD 1.10, 95% Cl -7.16 to 9.36), I² statistic = 74%, 4 trials, 202 participants, very low quality of evidence).
Authors' conclusions: There is insufficient evidence to support AT III substitution in any category of critically ill participants including the subset of patients with sepsis and DIC. We did not find a statistically significant effect of AT III on mortality, but AT III increased the risk of bleeding events. Subgroup analyses performed according to duration of intervention, length of follow-up, different patient groups, and use of adjuvant heparin did not show differences in the estimates of intervention effects. The majority of included trials were at high risk of bias (GRADE; very low quality of evidence for most of the analyses). Hence a large RCT of AT III is needed, without adjuvant heparin among critically ill patients such as those with severe sepsis and DIC, with prespecified inclusion criteria and good bias protection.
Conflict of interest statement
Mikkel Allingstrup declares that there are no conflicts of interest Jørn Wetterslev declares that he is a member of the task force on trial sequential analysis (TSA) at Copenhagen Trial Unit developing and programming TSA (please see
Figures

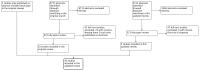
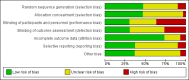
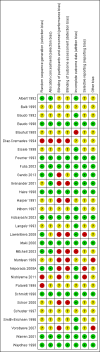
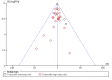























Update of
-
Antithrombin III for critically ill patients.Cochrane Database Syst Rev. 2008 Jul 16;(3):CD005370. doi: 10.1002/14651858.CD005370.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2016 Feb 08;2:CD005370. doi: 10.1002/14651858.CD005370.pub3. PMID: 18646125 Updated.
References
References to studies included in this review
Albert 1992 {published data only}
-
- Albert J, Blomqvist H, Gardlund B, Jakobsson J, Svensson J, Blomback M. Effect of antithrombin concentrate on haemostatic variables in critically ill patients. Acta Anaesthesiologica Scandinavia 1992;36(8):745‐52. [MEDLINE: ] - PubMed
Balk 1995 {published data only}
-
- Balk RA, Bedrosian C, McCormick L, Baughman J, Eisele B, Keinecke HO, et al. Prospective double blind placebo‐controlled trial of ATIII substitution in sepsis. Intensive Care Medicine 1995;21 Suppl 1:17.
Baudo 1992 {published data only}
-
- Baudo F, DeGasperi A, Cataldo F, Caimi TM, Cattaneo D, Redaelli R, et al. Antithrombin III supplementation during orthotopic liver transplantation in cirrhotic patients: a randomized trial. Thrombosis Research 1992;68(4‐5):409‐16. [MEDLINE: ] - PubMed
Baudo 1998 {published data only}
-
- Baudo F, Caimi TM, Cataldo F, Ravizza A, Arlati S, Casella G, et al. Antithrombin III (ATIII) replacement therapy in patients with severe sepsis and/or postsurgical complications: a controlled double‐blind, randomized, multicenter study. Intensive Care Medicine 1998;24(4):336‐42. [MEDLINE: ] - PubMed
Blauhut 1985 {published data only}
-
- Blauhut B, Kramar H, Vinazzer H, Bergmann H. Substitution of antithrombin III in shock and DIC: a randomized study. Thrombosis Research 1985;39(1):81‐9. [MEDLINE: ] - PubMed
Diaz‐Cremades 1994 {published data only}
-
- Diaz‐Cremades JM, Lorenzo R, Sánchez M, Moreno MJ, Alsar MJ, Bosch JM, et al. Use of antithrombin III in critical patients. Intensive Care Medicine 1994;20(8):577‐80. [MEDLINE: ] - PubMed
Eisele 1998 {published data only}
-
- Eisele B, Lamy M, Thijs LG, Keinecke HO, Schuster HP, Matthias FR, et al. Antithrombin III in patients with severe sepsis. A randomized, placebo‐controlled, double‐blind multicenter trial plus a meta‐analysis on all randomized, placebo‐controlled, double‐blind trials with antithrombin III in severe sepsis.. Intensive Care Medicine 1998;24(7):663‐72. [MEDLINE: ] - PubMed
Fourrier 1993 {published data only}
-
- Fourrier F, Chopin C, Huart JJ, Runge I, Caron C, Goudemand J. Double‐blind, placebo‐controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 1993;104(3):882‐8. [MEDLINE: ] - PubMed
Fulia 2003 {published data only}
-
- Fulia F, Cordaro S, Meo P, Gitto P, Gitto E, Trimarchi G, et al. Can the administration of antithrombin III decrease the risk of cerebral hemorrhage in premature infants?. Biology of the Neonate 2003;83(1):1‐5. [MEDLINE: ] - PubMed
Gando 2013 {published data only}
-
- Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group for the JAAM DIC Antithrombin Trial (JAAMDICAT). A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Critical Care 2013;17(6):297. [PUBMED: 24342495] - PMC - PubMed
-
- UMIN‐CTR Clinical Trial. Trial registration. upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=bro... (accessed 21 April 2015). [https://]
Grenander 2001 {published data only}
-
- Grenander A, Bredbacka S, Rydvall A, Aroch R, Edner G, Koskinen LO, et al. Antithrombin treatment in patients with traumatic brain Injury; a pilot study. Journal of Neurosurgical Anesthesiology 2001;13(1):49‐56. [MEDLINE: ] - PubMed
Haire 1998 {published data only}
-
- Haire WD, Ruby EI, Stephens LC, Reed E, Tarantolo SR, Pavletic ZS, et al. A prospective randomized double‐blind trial of antithrombin III concentrate in the treatment of multiple‐organ dysfunction syndrome during hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 1998;4(3):142‐50. [MEDLINE: ] - PubMed
Harper 1991 {published data only}
-
- Harper PL, Williamson L, Park G, Smith JK, Carrell RW. A pilot study of antithrombin replacement in intensive care management: the effects on mortality, coagulation and renal function. Transfusion Medicine 1991;1(2):121‐8. [MEDLINE: ] - PubMed
Inthorn 1997 {published data only}
-
- Hoffmann JN, Mühlbayer D, Jochum M, Inthorn D. Effect of long‐term and high‐dose antithrombin supplementation on coagulation and fibrinolysis in patients with severe sepsis. Critical Care Medicine 2004;32(9):1851‐9. [MEDLINE: ] - PubMed
-
- Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Antithrombin III supplementation in severe sepsis: beneficial effects on organ dysfunction. Shock 1997;8(5):328‐34. [MEDLINE: ] - PubMed
-
- Inthorn D, Hoffmann JN, Hartl WH, Mühlbayer D, Jochum M. Effect of antithrombin III supplementation on inflammatory response in patients with severe sepsis. Shock 1998;10(2):90‐6. [MEDLINE: ] - PubMed
Kobayashi 2003 {published data only}
-
- Kobayashi T, Terao T, Ikenoue T, Sameshima H, Nakabayashi M, Kajiwara Y, et al. BI 51 017 Study Group. Treatment of severe preeclampsia with antithrombin concentrate: results of a prospective feasibility study. Seminars in Thrombosis and Hemostasis 2003;29(6):645‐52. [MEDLINE: ] - PubMed
Langely 1993 {published data only}
-
- Langley PG, Hughes RD, Forbes A, Keays R, Williams R. Controlled trial of antithrombin III supplementation in fulminant hepatic failure. Journal of Hepatology 1993;17(3):326‐31. [MEDLINE: ] - PubMed
Lavrentieva 2008 {published data only}
-
- Lavrentieva A, Kontakiotis T, Bitzani M, Parlapani A, Thomareis O, Scourtis H, et al. The efficacy of antithrombin administration in the acute phase of burn injury.. Thrombosis and Haemostasis 2008;100(2):286–90. [PUBMED: 18690349] - PubMed
Maki 2000 {published data only}
-
- Maki M, Kobayashi T, Terao T, Ikenoue T, Satoh K, Nakabayashi M, et al. BI51.017 Study Group. Antithrombin therapy for severe preeclampsia: results of a double‐blind, randomized, placebo‐controlled trial. Thrombosis and Haemostasis 2000;84(4):583‐90. [MEDLINE: ] - PubMed
-
- Sameshima H, Kodama Y, Ikenoue T, Kajiwara Y. Antithrombin improves fetal condition in women with severe pre‐eclampsia before 32 weeks of gestation;a randomized, double‐blind, placebo‐controlled trial. Journal of Obstetrics and Gynaecology Research 2008;34(1):34–9. [PUBMED: 18226126] - PubMed
Mitchell 2003 {published data only}
-
- Mitchell L, Andrew M, Hanna K, Abshire T, Halton J, Wu J, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l‐asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thrombosis and Haemostasis 2003;90(2):235‐44. [MEDLINE: ] - PubMed
-
- Mitchell LG, Andrew M, Hanna K, Abshire T, Halton J, Anderson R, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L‐asparaginase ‐ Results of the prophylactic antithrombin replacement in kids with acute lymphoblastic leukemia treated with asparaginase (PARKAA) study. Cancer 2003;97(2):508‐16. [MEDLINE: ] - PubMed
Muntean 1989 {published data only}
-
- Muntean W, Rossegger H. Antithrombin III concentrate in preterm infants with IRDS: an open, controlled, randomized clinical trial (abstract). Thrombosis and Haemostasis 1989;62:288.
Neporada 2008A {published and unpublished data}
-
- Neporada E, Vorobyeva N, Nedashkovsky E. Antithrombin III deficiency correction in patients with disseminated intravascular coagulation. Obshaya Reanimatologia 2008;5:49‐54.
Nishiyama 2011 {published data only}
-
- Nishiyama T, Kohno Y, Koishi K. Effects of antithrombin and gabexate mesilate on disseminated intravascular coagulation: a preliminary study.. American Journal of Emergency Medicine 2012;30(7):1219–23. [PUBMED: 22204993] - PubMed
Palareti 1995 {published data only}
-
- Palareti G, Legnani C, Coccheri S, Ridolfi L, Baudo F, Caimi TM, et al. Laboratory effects of antithrombin‐III (ATIII) replacement in patients with sepsis and or postsurgical complications requiring hemodynamic and or respiratory support ‐ a controlled, double‐blind, randomized multicenter study. Thrombosis and Haemostasis 1995;73(6):1251.
Schmidt 1998 {published data only}
-
- Schmidt B, Gillie P, Mitchell L, Andrew M, Caco C, Roberts R. A placebo‐controlled randomized trial of antithrombin therapy in neonatal respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 1998;158(2):470‐6. [MEDLINE: ] - PubMed
Schorr 2000 {published data only}
-
- Schorr M, Siebeck M, Zugel N, Welcker K, Gippner‐Steppert C, Czwienzek E, et al. Antithrombin III and local serum application: adjuvant therapy in peritonitis. European Journal of Clinical Investigation 2000;30(4):359‐66. [MEDLINE: ] - PubMed
Schuster 1997 {published data only}
-
- Schuster HP, Eisele B, Keinecke HO, Heinrichs H, Mescheder A, Knaub S. S‐AT III study: antithrombin III in patients with sepsis. Intensive Care Medicine 1997;23 Suppl 1:76.
Smith‐Erichsen 1996 {published data only}
-
- Smith‐Erichsen N, Aasen AO, Kongsgaard UE, Bakka A, Bergen A, Bjerkelund CE, et al. The effect of antithrombin III substitution therapy on components of the plasma protease systems in surgical patients. Clinical Intensive Care 1996;7:291‐6.
Vorobyeva 2007 {published data only}
-
- Vorob'eva N, Neporada E, Turundaevskaia O, Mel'nikova G. Place of antithrombin III concentrate in the intensive care of disseminated intravascular coagulation. Anesteziologiia i Reanimatologiia 2007;2:42‐4. [PUBMED: 17563999] - PubMed
Warren 2001 {published data only}
-
- Angstwurm M, Hoffmann J, Ostermann H, Frey L, Spannagl M. Severe sepsis and disseminated intravascular coagulation. Supplementation with antithrombin [Schwere sepsisund disseminierte intravasale erinnung. Substitution von antithrombin]. Anaesthesist 2009;58(2):171–9. [PUBMED: 19189066] - PubMed
-
- Eid A, Wiedermann CJ, Kinasewitz GT. Early administration of high‐dose antithrombin in severe sepsis: single center results from the KyberSept‐trial. Anesthesia and Analgesia 2008;107(5):1633‐8. [PUBMED: 18931224] - PubMed
-
- Hoffmann JN, Wiedermann CJ, Juers M, Ostermann H, Kienast J, Briegel J, et al. Benefit/risk profile of high‐dose antithrombin in patients with severe sepsis treated with and without concomitant heparin. Thrombosis and Haemostasis 2006;95(5):850‐6. [MEDLINE: ] - PubMed
-
- Kienast J, Juers M, Wiedermann CJ, Hoffmann JN, Ostermann H, Strauss R, et al. Treatment effects of high‐dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. Journal of Thrombosis and Haemostasis 2006;4(1):90‐7. [MEDLINE: ] - PubMed
Waydhas 1998 {published data only}
-
- Waydhas C, Nast‐Kolb D, Gippner‐Steppert C, Trupka A, Pfundstein C, Schweiberer L, et al. High‐dose antithrombin III treatment of severely injured patients: results of a prospective study. Journal of Trauma, Injury, Infection and Critical Care 1998;45(5):931‐40. [MEDLINE: ] - PubMed
References to studies excluded from this review
Aibiki 2006 {published data only}
-
- Aibiki M, Fukuoka N, Nishiyama T, Maekawa S, Shirakawa Y. Differences in antithrombin III activities by administration method in critical patients with disseminated intravascular coagulation: a pharmacokinetic study. Shock 2007;28(2):141‐7. [PUBMED: 17515857] - PubMed
Dietrich 2013 {published data only}
-
- Dietrich W, Busley R, Spannagl M, Braun S, Schuster T, Lison S. The influence of antithrombin substitution on heparin sensitivity and activation of hemostasis during coronary artery bypass graft surgery: a dose‐finding study. Anesthesia and Analgesia 2013;116(6):1223‐30. [PUBMED: 23408673] - PubMed
Doi 2012 {published data only}
-
- Doi T, Kozawa O, Enomoto Y, Ogura S. Analysis of the anti‐inflammatory effects of antithrombin III on human platelets. 7th Congress of the International Federation of Shock Societies ‐ 35th Annual Conference on Shock Miami Beach, FL United States 2012;1:198.
Hoffmann 2010 {published data only}
-
- Hoffmann JN, Fertmann JM, Illner W, Arbogast H, Land H, Jauch K. High‐dose antithrombin can not improve kidney function despite improved graft perfusion in human kidney transplantation‐final results of a prospective, controlled trial. 23rd International Congress of the Transplantation Society, TTS 2010 Vancouver, BC Canada. 2010; Vol. 1:1882.
Ilias 2000 {published data only}
-
- Ilias W, List W, Decruyenaere J, Lignian H, Knaub S, Schindel F, et al. Antithrombin III in patients with severe sepsis: a pharmacokinetic study. Intensive Care Medicine 2000;26(6):704‐15. [MEDLINE: ] - PubMed
Jochum 1995 {published and unpublished data}
-
- Jochum M. Influence of high‐dose antithrombin concentrate therapy on the release of cellular proteinases, cytokines, and soluble adhesion molecules in acute inflammation. Seminars in Hematology 1995;32 Suppl 2(4):19‐32. [MEDLINE: ] - PubMed
Kanbak 2011 {published data only}
-
- Kanbak M, Oc B, Salman MA, Ocal T, Oc M. Peroperative effects of fresh frozen plasma and antithrombin III on heparin sensitivity andcoagulation during nitroglycerine infusion in coronary artery bypass surgery. Blood Coagulation and Fibrinolysis 2011;22(7):593‐9. [PUBMED: 21799398] - PubMed
Kim 2013 {published data only}
-
- Kim KA, Lim YY, Kim SH, Park JY. Bioequivalence of two intravenous formulations of antithrombin III: a two‐way crossover study in healthy Korean subjects. Clinical Therapeutics 2013;35(11):1752‐61. [PUBMED: 24094463] - PubMed
Korninger 1987 {published data only}
-
- Korninger C, Klepteko W, Miholic J, Schwarz C, Lechner K. Randomized trial of antithrombin‐III versus placebo in patients undergoing peritoneo‐venous shunt operation. Thrombosis and Haemostasis 1987;58(1):426.
Leitner 2006 {published data only}
-
- Leitner JM, Firbas C, Mayr FB, Reiter RA, Steinlechner B, Jilma B. Recombinant human antithrombin inhibits thrombin formation and interleukin 6 release in human endotoxemia. Clinical Pharmacology and Therapeutics 2006;79(1):23‐34. [MEDLINE: ] - PubMed
Maki 1987 {published data only}
-
- Maki M, Terao T, Ikenoue T, Takemura T, Sekiba K, Shirakawa K, et al. Clinical evaluation of antithrombin III concentrate (BI 6.013) for disseminated intravascular coagulation in obstetrics. Well‐controlled multicenter trial. Gynecology and Obstetric Investigation 1987;23(4):230‐40. [MEDLINE: ] - PubMed
Mayumi 2011 {published data only}
-
- Mayumi T, Suzuki S, Yamamoto T, Ichikawa T, Onodera M, Tsuzuki M, et al. Levels of antithrombin activity after antithrombin administration indicate prognosis of septic DIC patients. Shock Society 2011; Vol. 1:www.shocksociety.org/assets/docs/shock%202011%20oral%20abstracts.pdf.
Neporada 2008B {unpublished data only}
-
- Neporada E, Turundayevskaya O, Vorobyeva N. Antithrombin III concentrate in comparison with FFP in patients with disseminated intravascular coagulation [КОНЦЕНТРАТ АНТИТРОМБИНА III В СРАВНЕНИИ СО СВЕЖЕЗАМОРОЖЕННОЙ ПЛАЗМОЙ ПРИ СИНДРОМЕ ДИССЕМИНИРОВАННОГО ВНУТРИСОСУДИСТОГО СВЕРТЫВАНИЯ]. Human Ecology 2008;7:47‐51.
Nishiyama 2006 {published data only}
-
- Nishiyama T. Antithrombin can modulate coagulation, cytokine production, and expression of adhesion molecules in abdominal aortic aneurysm repair surgery. Anesthesia and Analgesia 2006;102(4):1007‐11. [MEDLINE: ] - PubMed
Paparella 2014 {published data only}
-
- NCT01201070. Trial registration. clinicaltrials.gov/ct2/show/NCT01201070 (accessed 21 April 2015).
-
- Paparella D, Rotunno C, Palo M, Finamore S, Guida P, Rubino G, et al. Antithrombin administration in patients with low antithrombin values after cardiac surgery: a randomized controlled trial. Annals of Thoracic Surgery 2014;97(4):1207‐13. [PUBMED: 24507941] - PubMed
Paternoster 2000 {published data only}
-
- Paternoster DM, Fusco D, Tambuscio B. Treatment with antithrombin III (AT III) concentrate in pre‐eclampsia. International Journal of Gynaecology and Obstetrics 2000;71(2):175‐6. [MEDLINE: ] - PubMed
Paternoster 2004 {published data only}
-
- Paternoster DM, Fantinato S, Manganelli F. Efficacy of AT in pre‐eclampsia: a case‐control prospective trial. Thrombosis and Haemostasis 2004;91(2):283‐9. [MEDLINE: ] - PubMed
Ranucci 2013 {published data only}
-
- Ranucci M, Baryshnikova E, Crapelli GB, Woodward MK, Paez A, Pelissero G. Preoperative antithrombin supplementation in cardiac surgery: a randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery 2013;145(5):1393‐9. [PUBMED: 23102903] - PubMed
Sawamura 2009 {published data only}
-
- Sawamura A, Gando S, Hayakawa M, Hoshino H, Kubota N, Sugano M. Effects of antithrombin III in patients with disseminated intravascular coagulation diagnosed by newly developed diagnostic criteria for critical illness. Clinical and Applied Thrombosis/Hemostasis 2009;15(5):561‐6. [PUBMED: 18840625] - PubMed
Scherer 1997 {published data only}
-
- Scherer R, Kabatnik M, Erhard J, Peters J. The influence of antithrombin III (AT III) substitution to supranormal activities on systemic procoagulant turnover in patients with end‐stage chronic liver disease. Intensive Care Medicine 1997;23(11):1150‐8. [MEDLINE: ] - PubMed
Shimada 1994 {published data only}
-
- Shimada M, Matsumata T, Kamakura T, Hayashi H, Urata K, Sugimachi K. Modulation of coagulation and fibrinolysis in hepatic resection: a randomized prospective control study using antithrombin III concentrates. Thrombosis Research 1994;74(2):105‐14. [MEDLINE: ] - PubMed
Terao 1989 {published data only}
-
- Terao T, Kobayashi T, Imai N, Oda H, Karasawa T. Pathological state of the coagulatory and fibrinolytic system in preeclampsia and the possibility of its treatment. Asia‐Oceania Journal of Obstetrics and Gynaecology 1988;15(1):25‐32. [MEDLINE: ] - PubMed
Valsecchi 2008 {published data only}
-
- Valsecchi L, D’Angelo A. High‐dose antithrombin supplementation in early pre‐eclampsia: a double‐blind, placebo controlled study (on behalf of the ATIII‐EPAS study group). Journal of Thrombosis and Haemostasis 2009;7:297‐8.
Vinazzer 1995 {published data only}
-
- Vinazzer HA. Antithrombin III in shock and disseminate intravascular coagulation. Clinical and Applied Thrombosis/Hemostasis 1995;1(1):62‐5.
References to ongoing studies
D'angelo 2005 {published data only}
-
- D'angelo A, Valsecchi L. A double‐blind trial of high‐dose antithrombin supplementation in early pre‐eclampsia. Thrombosis Research 2005;115(Suppl 1):117. - PubMed
Additional references
Altman 2003
Becker 2000
-
- Becker BF, Heindl B, Kupatt C, Zahler S. Endothelial function and hemostasis. Zeitschrift fur Kardiologie 2000;89(3):160‐7. [MEDLINE: ] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [MEDLINE: ] - PubMed
Chan 2004
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [MEDLINE: ] - PubMed
Diaz 2015
Fourrier 2000
-
- Fourrier F, Jourdain M, Tournoys A. Clinical trial results with antithrombin III in sepsis. Critical Care Medicine 2000;28(9 Suppl):S38‐43. [MEDLINE: ] - PubMed
Gotzsche 2000
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;15(11):1539‐58. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [DOI: 10.1002/9780470712184] - DOI
Hutton 2000
-
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series 2000;49(3):359‐70. [DOI: 10.1111/1467-9876.00197] - DOI
Lan 1983
-
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70(3):659‐63.
Larsson 2001
-
- Larsson H, Åkerud P, Nordling K, Raub‐Segall E, Claesson‐Welsh L, Bjork I. A novel anti‐angiogenic form of antithrombin with retained proteinase binding ability and heparin affinity. Journal of Biological Chemistry 2001;276(15):11996‐2002. [MEDLINE: ] - PubMed
Levi 2004
-
- Levi M, Jonge E, Poll T. New treatment strategies for disseminated intravascular coagulation based on current understanding of the pathophysiology. Annals of Medicine 2004;36(1):41‐9. - PubMed
Mayr 2014
Opal 2002
-
- Opal SM, Kessler CM, Roemisch J, Knaub S. Antithrombin, heparin, and heparan sulfate. Critical Care Medicine 2002;30(5 Suppl):S325‐31. [MEDLINE: ] - PubMed
Opal 2003
-
- Opal SM. Clinical trial design and outcomes in patients with severe sepsis. Shock 2003;20(4):295‐302. [MEDLINE: ] - PubMed
Periti 2000
-
- Periti P. Current treatment of sepsis and endotoxaemia. Expert opinion of Pharmacotherapy 2000;1(6):1203‐17. [MEDLINE: ] - PubMed
Pogue 1997
-
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: Utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93. - PubMed
Pogue 1998
-
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. - PubMed
Polderman 2004
-
- Polderman KH, Girbes ARJ. Drug intervention trials in sepsis: divergent results. Lancet 2004;363(9422):1721‐3. [MEDLINE: ] - PubMed
RevMan 5.3 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rublee 2003
Tagami 2014
-
- Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis‐associated disseminated intravascular coagulation: an observational nationwide study. Journal of Thrombosis and Haemostasis September 2014;12(9):1470–9. [PUBMED: 24943516] - PubMed
Tagami 2015
-
- Tagami T, Matsui H, Fushimi K, Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patientsafter abdominal sepsis. Thrombosis and Haemostasis 2015;114(3):537‐45. [PUBMED: 25948492] - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [MEDLINE: ] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] - PubMed
Wetterslev 2009
Wiedermann 2002
-
- Wiedermann CJ, Römisch J. The anti‐inflammatory actions of antithrombin ‐ a review. Acta Medica Austriaca 2002;29(3):89‐92. [MEDLINE: ] - PubMed
Wunsch 2008
-
- Wunsch H, Angus D, Harrison D, Collange O, Fowler R, Hoste E, et al. Variation in critical care services across North America and Western Europe. Critical Care Medicine 2008;36(10):2787‐93. [PUBMED: 18766102] - PubMed
Zarychanski 2015
-
- Zarychanski R, Abou‐Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, et al. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Critical Care Medicine 2015;43(3):511–8. [PUBMED: 25493972] - PubMed
References to other published versions of this review
Afshari 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

