Cyanobacteria use micro-optics to sense light direction
- PMID: 26858197
- PMCID: PMC4758948
- DOI: 10.7554/eLife.12620
Cyanobacteria use micro-optics to sense light direction
Abstract
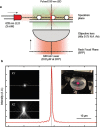
Bacterial phototaxis was first recognized over a century ago, but the method by which such small cells can sense the direction of illumination has remained puzzling. The unicellular cyanobacterium Synechocystis sp. PCC 6803 moves with Type IV pili and measures light intensity and color with a range of photoreceptors. Here, we show that individual Synechocystis cells do not respond to a spatiotemporal gradient in light intensity, but rather they directly and accurately sense the position of a light source. We show that directional light sensing is possible because Synechocystis cells act as spherical microlenses, allowing the cell to see a light source and move towards it. A high-resolution image of the light source is focused on the edge of the cell opposite to the source, triggering movement away from the focused spot. Spherical cyanobacteria are probably the world's smallest and oldest example of a camera eye.
Keywords: Cyanobacteria; Micro-optics; Phototaxis; Signal transduction; Synechocystis sp PCC6803; Thermosynechococcus elongatus; biophysics; infectious disease; microbiology; structural biology.
Plain language summary
Cyanobacteria are blue-green bacteria that are abundant in the environment. Cyanobacteria in the oceans are among the world’s most important oxygen producers and carbon dioxide consumers. Synechocystis is a spherical single-celled cyanobacterium that measures about three thousandths of a millimetre across. Because Synechocystis needs sunlight to produce energy, it is important for it to find places where the light is neither too weak nor too strong. Unlike some bacteria, Synechocystis can’t swim, but it can crawl across surfaces. It uses this ability to move to places where the light conditions are better. It was already known that Synechocystis cells move towards a light source that is shone at them from one side, which implies that the cyanobacteria can “see” where the light is. But how can such a tiny cell accurately detect where light is coming from? Schuergers et al. tracked how Synechocystis moved in response to different light conditions, and found that the secret of “vision” in these cyanobacteria is that the cells act as tiny spherical lenses. When a light is shone at the cell, an image of the light source is focused at the opposite edge of the cell. Light-detecting molecules called photoreceptors respond to the focused image of the light source, and this provides the information needed to steer the cell towards the light. Although the details are different, and although a Synechocystis cell is in terms of volume about 500 billion times smaller than a human eyeball, vision in Synechocystis actually works by principles similar to vision in humans. Schuergers et al.’s findings open plenty of further questions, as other types of bacteria may also act as tiny lenses. More also remains to be learnt about how the cyanobacteria process visual information.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Comment in
-
Phototaxis: Life in focus.Elife. 2016 Feb 9;5:e14169. doi: 10.7554/eLife.14169. Elife. 2016. PMID: 26880564 Free PMC article.
-
Comparative Vision: Can Bacteria Really See?Curr Biol. 2016 May 9;26(9):R369-71. doi: 10.1016/j.cub.2016.03.025. Curr Biol. 2016. PMID: 27166697
References
-
- Agostinelli C, Lund U. R package “circular”: circular statistics (version 0.4-7). at. 2013. https://r-forge.r-project.org/projects/circular/ https://r-forge.r-project.org/projects/circular/
-
- Alam M, Oesterhelt D. Morphology, function and isolation of halobacterial flagella. Journal of Molecular Biology. 1984;176:459–475. - PubMed
-
- Berg HC, Brown DA. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972;239:500–504. - PubMed
-
- Bhaya D, Bianco NR, Bryant D, Grossman A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Molecular Microbiology. 2000;37:941–951. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

