Inhibition of the Formation of the Spf1p Phosphoenzyme by Ca2
- PMID: 26858246
- PMCID: PMC4817200
- DOI: 10.1074/jbc.M115.695122
Inhibition of the Formation of the Spf1p Phosphoenzyme by Ca2
Abstract
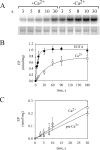
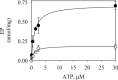
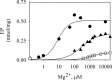
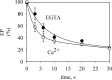
P5-ATPases are important for processes associated with the endosomal-lysosomal system of eukaryotic cells. In humans, the loss of function of P5-ATPases causes neurodegeneration. In the yeastSaccharomyces cerevisiae, deletion of P5-ATPase Spf1p gives rise to endoplasmic reticulum stress. The reaction cycle of P5-ATPases is poorly characterized. Here, we showed that the formation of the Spf1p catalytic phosphoenzyme was fast in a reaction medium containing ATP, Mg(2+), and EGTA. Low concentrations of Ca(2+)in the phosphorylation medium decreased the rate of phosphorylation and the maximal level of phosphoenzyme. Neither Mn(2+)nor Mg(2+)had an inhibitory effect on the formation of the phosphoenzyme similar to that of Ca(2+) TheKmfor ATP in the phosphorylation reaction was ∼1 μmand did not significantly change in the presence of Ca(2+) Half-maximal phosphorylation was attained at 8 μmMg(2+), but higher concentrations partially protected from Ca(2+)inhibition. In conditions similar to those used for phosphorylation, Ca(2+)had a small effect accelerating dephosphorylation and minimally affected ATPase activity, suggesting that the formation of the phosphoenzyme was not the limiting step of the ATP hydrolytic cycle.
Keywords: ATPase; P-ATPase; P5-ATPase; ion pump; phosphoryl transfer; phosphorylation; protein phosphorylation; transporter.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Reduction of the P5A-ATPase Spf1p phosphoenzyme by a Ca2+-dependent phosphatase.PLoS One. 2020 Apr 30;15(4):e0232476. doi: 10.1371/journal.pone.0232476. eCollection 2020. PLoS One. 2020. PMID: 32353073 Free PMC article.
-
The effect of metal ions on the Spf1p P5A-ATPase. High sensitivity to irreversible inhibition by zinc.Arch Biochem Biophys. 2022 Dec 15;732:109450. doi: 10.1016/j.abb.2022.109450. Epub 2022 Oct 31. Arch Biochem Biophys. 2022. PMID: 36328152
-
The Spf1p P5A-ATPase "arm-like" domain is not essential for ATP hydrolysis but its deletion impairs autophosphorylation.Biochem Biophys Res Commun. 2021 Jul 23;563:113-118. doi: 10.1016/j.bbrc.2021.05.054. Epub 2021 Jun 1. Biochem Biophys Res Commun. 2021. PMID: 34087682
-
Towards defining the substrate of orphan P5A-ATPases.Biochim Biophys Acta. 2015 Mar;1850(3):524-35. doi: 10.1016/j.bbagen.2014.05.008. Epub 2014 May 14. Biochim Biophys Acta. 2015. PMID: 24836520 Review.
-
New wine in old bottles: current progress on P5 ATPases.FEBS J. 2022 Dec;289(23):7304-7313. doi: 10.1111/febs.16172. Epub 2021 Sep 7. FEBS J. 2022. PMID: 34449980 Review.
Cited by
-
ATP hydrolytic activity of purified Spf1p correlate with micellar lipid fluidity and is dependent on conserved residues in transmembrane helix M1.PLoS One. 2022 Oct 20;17(10):e0274908. doi: 10.1371/journal.pone.0274908. eCollection 2022. PLoS One. 2022. PMID: 36264897 Free PMC article.
-
Highly exposed segment of the Spf1p P5A-ATPase near transmembrane M5 detected by limited proteolysis.PLoS One. 2021 Jan 28;16(1):e0245679. doi: 10.1371/journal.pone.0245679. eCollection 2021. PLoS One. 2021. PMID: 33507968 Free PMC article.
-
Copper resistance in the cold: Genome analysis and characterisation of a PIB-1 ATPase in Bizionia argentinensis.Environ Microbiol Rep. 2024 Aug;16(4):e13278. doi: 10.1111/1758-2229.13278. Environ Microbiol Rep. 2024. PMID: 38943264 Free PMC article.
-
Reduction of the P5A-ATPase Spf1p phosphoenzyme by a Ca2+-dependent phosphatase.PLoS One. 2020 Apr 30;15(4):e0232476. doi: 10.1371/journal.pone.0232476. eCollection 2020. PLoS One. 2020. PMID: 32353073 Free PMC article.
References
-
- Palmgren M. G., Axelsen K. B. (1998) Evolution of P-type ATPases. Biochim. Biophys. Acta 1365, 37–45 - PubMed
-
- Sørensen D. M., Buch-Pedersen M. J., and Palmgren M. G. (2010) Structural divergence between the two subgroups of P5 ATPases. Biochim. Biophys. Acta 1797, 846–855 - PubMed
-
- Perrett R. M., Alexopoulou Z., and Tofaris G. K. (2015) The endosomal pathway in Parkinson's disease. Mol. Cell Neurosci. 66, 21–28 - PubMed
-
- Suzuki C., and Shimma Y. I. (1999) P-type ATPase Spf1 mutants show a novel resistance mechanism for the killer toxin SMKT. Mol. Microbiol. 32, 813–823 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

