First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction
- PMID: 26858336
- PMCID: PMC5470113
- DOI: 10.1200/JCO.2015.63.6530
First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction
Abstract
Purpose: Light chain (AL) amyloidosis is caused by the accumulation of misfolded proteins, which induces the dysfunction of vital organs. NEOD001 is a monoclonal antibody targeting these misfolded proteins. We report interim data from a phase I/II dose-escalation/expansion study of NEOD001 in patients with AL amyloidosis and persistent organ dysfunction (NCT01707264).
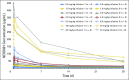
Patients and methods: Patients who had completed at least one previous anti-plasma cell-directed therapy, had partial hematologic response or better, and had persistent organ dysfunction received NEOD001 intravenously every 28 days. Dose levels of 0.5, 1, 2, 4, 8, 16, and 24 mg/kg were evaluated (3 + 3 study design). Primary objectives were to determine the maximum tolerated dose and the recommended dose for future studies and to evaluate safety/tolerability. Secondary and exploratory objectives included pharmacokinetics, immunogenicity, and organ responses on the basis of published consensus criteria.
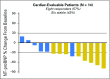
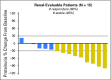
Results: Twenty-seven patients were enrolled in seven cohorts (dose-escalation component). No drug-related serious adverse events (AEs), discontinuations because of drug-related AEs, dose-limiting toxicities, or antidrug antibodies were reported. The most frequent AEs were fatigue, upper respiratory tract infection, cough, and dyspnea. Recommended dosing was 24 mg/kg. Pharmacokinetics support intravenous dosing every 28 days. Of 14 cardiac-evaluable patients, eight (57%) met the criteria for cardiac response and six (43%) had stable disease. Of 15 renal-evaluable patients, nine (60%) met the criteria for renal response and six (40%) had stable disease.
Conclusion: Monthly infusions of NEOD001 were safe and well tolerated. Recommended future dosing was 24 mg/kg. Organ response rates compared favorably with those reported previously for chemotherapy. A phase II expansion is ongoing. A global phase III study (NCT02312206) has been initiated. Antibody therapy targeting misfolded proteins is a potential new therapy for the management of AL amyloidosis.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
References
-
- Sanchorawala V. Light-chain (AL) amyloidosis: Diagnosis and treatment. Clin J Am Soc Nephrol. 2006;1:1331–1341. - PubMed
-
- Merlini G, Wechalekar AD, Palladini G. Systemic light chain amyloidosis: An update for treating physicians. Blood. 2013;121:5124–5130. - PubMed
-
- Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79:319–328. - PubMed
-
- Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

