Determinants of Early Mortality Among 37,568 Patients With Colon Cancer Who Participated in 25 Clinical Trials From the Adjuvant Colon Cancer Endpoints Database
- PMID: 26858337
- PMCID: PMC4933130
- DOI: 10.1200/JCO.2015.65.1158
Determinants of Early Mortality Among 37,568 Patients With Colon Cancer Who Participated in 25 Clinical Trials From the Adjuvant Colon Cancer Endpoints Database
Abstract
Purpose: Factors associated with early mortality after surgery and treatment with adjuvant chemotherapy in colon cancer are poorly understood. We aimed to characterize the determinants of early mortality in a large cohort of colon cancer trial participants.
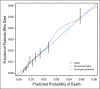
Methods: A pooled analysis of 37,568 patients in 25 randomized trials of adjuvant systemic therapy was conducted. Multivariable logistic regression models with several definitions of early mortality (30, 60, and 90 days, and 6 months) were constructed, adjusting for clinically and statistically significant variables. A nomogram for 6-month mortality was developed and validated.
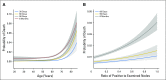
Results: Median age among patients was 61 years, patient demographics included 54% men and 90% White, 29% and 71% had stage II and III disease, respectively, and 79%, 20%, and 1% had an Eastern Cooperative Oncology Group performance status (PS) of 0, 1, and ≥ 2, respectively. Early mortality was low: 0.3% at 30 days, 0.6% at 60 days, 0.8% at 90 days, and 1.4% at 6 months. Of those patients who died by 6 months post-random assignment, 40% had documented disease recurrence prior to death. Early disease recurrence was associated with a markedly increased risk of death during the first 6 months post-treatment (hazard ratio, 82.6; 95%CI, 66.9 to 102.1). In prognostic analyses, advanced age, male sex, poorer PS, increasing ratio of positive to examined lymph nodes, earlier decade of enrollment, and higher tumor stage and grade predicted a greater likelihood of early mortality, whereas treatment received was not strongly predictive. A multivariable model for 6-month mortality showed strong optimism-adjusted discrimination (concordance index, 0.73) and calibration.
Conclusion: Early mortality was infrequent but more prevalent in patients with advanced age and a PS of ≥ 2, underscoring the need to carefully consider the risk-to-benefit ratio when making treatment decisions in these subgroups.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Big Data, Small Effects.J Clin Oncol. 2016 Apr 10;34(11):1170-1. doi: 10.1200/JCO.2015.65.8161. Epub 2016 Feb 16. J Clin Oncol. 2016. PMID: 26884573 No abstract available.
References
-
- Barrios CH, Werutsky G, Martinez-Mesa J. The global conduct of cancer clinical trials: Challenges and opportunities. Am Soc Clin Oncol Educ Book. 2015;35:e132–e139. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

