Structure of the Brd4 ET domain bound to a C-terminal motif from γ-retroviral integrases reveals a conserved mechanism of interaction
- PMID: 26858406
- PMCID: PMC4776507
- DOI: 10.1073/pnas.1516813113
Structure of the Brd4 ET domain bound to a C-terminal motif from γ-retroviral integrases reveals a conserved mechanism of interaction
Abstract
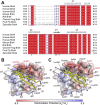
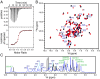
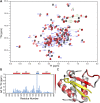
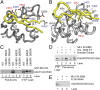
The bromodomain and extraterminal domain (BET) protein family are promising therapeutic targets for a range of diseases linked to transcriptional activation, cancer, viral latency, and viral integration. Tandem bromodomains selectively tether BET proteins to chromatin by engaging cognate acetylated histone marks, and the extraterminal (ET) domain is the focal point for recruiting a range of cellular and viral proteins. BET proteins guide γ-retroviral integration to transcription start sites and enhancers through bimodal interaction with chromatin and the γ-retroviral integrase (IN). We report the NMR-derived solution structure of the Brd4 ET domain bound to a conserved peptide sequence from the C terminus of murine leukemia virus (MLV) IN. The complex reveals a protein-protein interaction governed by the binding-coupled folding of disordered regions in both interacting partners to form a well-structured intermolecular three-stranded β sheet. In addition, we show that a peptide comprising the ET binding motif (EBM) of MLV IN can disrupt the cognate interaction of Brd4 with NSD3, and that substitutions of Brd4 ET residues essential for binding MLV IN also impair interaction of Brd4 with a number of cellular partners involved in transcriptional regulation and chromatin remodeling. This suggests that γ-retroviruses have evolved the EBM to mimic a cognate interaction motif to achieve effective integration in host chromatin. Collectively, our findings identify key structural features of the ET domain of Brd4 that allow for interactions with both cellular and viral proteins.
Keywords: BET family transcription factor; ET binding motif (EBM); NMR; protein–protein interaction; retroviral integration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Bromo- and extraterminal domain chromatin regulators serve as cofactors for murine leukemia virus integration.J Virol. 2013 Dec;87(23):12721-36. doi: 10.1128/JVI.01942-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049186 Free PMC article.
-
A common binding motif in the ET domain of BRD3 forms polymorphic structural interfaces with host and viral proteins.Structure. 2021 Aug 5;29(8):886-898.e6. doi: 10.1016/j.str.2021.01.010. Epub 2021 Feb 15. Structure. 2021. PMID: 33592170 Free PMC article.
-
Bimodal high-affinity association of Brd4 with murine leukemia virus integrase and mononucleosomes.Nucleic Acids Res. 2014 Apr;42(8):4868-81. doi: 10.1093/nar/gku135. Epub 2014 Feb 11. Nucleic Acids Res. 2014. PMID: 24520112 Free PMC article.
-
Targeting BET bromodomains for cancer treatment.Epigenomics. 2015;7(3):487-501. doi: 10.2217/epi.14.91. Epigenomics. 2015. PMID: 26077433 Review.
-
BET Bromodomain as a Target of Epigenetic Therapy.Chem Pharm Bull (Tokyo). 2016;64(6):540-7. doi: 10.1248/cpb.c16-00225. Chem Pharm Bull (Tokyo). 2016. PMID: 27250788 Review.
Cited by
-
Functional Analysis of the Replication Fork Proteome Identifies BET Proteins as PCNA Regulators.Cell Rep. 2019 Sep 24;28(13):3497-3509.e4. doi: 10.1016/j.celrep.2019.08.051. Cell Rep. 2019. PMID: 31553917 Free PMC article.
-
BET Bromodomain Inhibitors: Novel Design Strategies and Therapeutic Applications.Molecules. 2023 Mar 29;28(7):3043. doi: 10.3390/molecules28073043. Molecules. 2023. PMID: 37049806 Free PMC article. Review.
-
The Short Isoform of BRD4 Promotes HIV-1 Latency by Engaging Repressive SWI/SNF Chromatin-Remodeling Complexes.Mol Cell. 2017 Sep 21;67(6):1001-1012.e6. doi: 10.1016/j.molcel.2017.07.025. Epub 2017 Aug 24. Mol Cell. 2017. PMID: 28844864 Free PMC article.
-
Peptide Inhibitor Targeting the Extraterminal Domain in BRD4 Potently Suppresses Breast Cancer Both In Vitro and In Vivo.J Med Chem. 2024 Apr 25;67(8):6658-6672. doi: 10.1021/acs.jmedchem.4c00141. Epub 2024 Apr 3. J Med Chem. 2024. PMID: 38569135 Free PMC article.
-
Structure and function of retroviral integrase.Nat Rev Microbiol. 2022 Jan;20(1):20-34. doi: 10.1038/s41579-021-00586-9. Epub 2021 Jul 9. Nat Rev Microbiol. 2022. PMID: 34244677 Free PMC article. Review.
References
-
- McBride AA, McPhillips MG, Oliveira JG. Brd4: Tethering, segregation and beyond. Trends Microbiol. 2004;12(12):527–529. - PubMed
-
- Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282(18):13141–13145. - PubMed
-
- Yang Z, et al. Recruitment of P-TEFb for stimulation of transcriptional elongation by the bromodomain protein Brd4. Mol Cell. 2005;19(4):535–545. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

