Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites
- PMID: 26858414
- PMCID: PMC4776486
- DOI: 10.1073/pnas.1519730113
Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites
Abstract
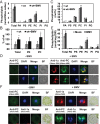
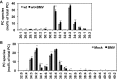
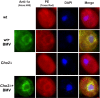
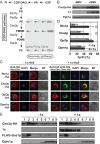
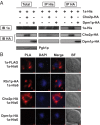
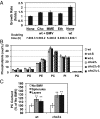
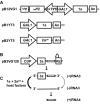
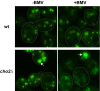
All positive-strand RNA viruses reorganize host intracellular membranes to assemble their viral replication complexes (VRCs); however, how these viruses modulate host lipid metabolism to accommodate such membrane proliferation and rearrangements is not well defined. We show that a significantly increased phosphatidylcholine (PC) content is associated with brome mosaic virus (BMV) replication in both natural host barley and alternate host yeast based on a lipidomic analysis. Enhanced PC levels are primarily associated with the perinuclear ER membrane, where BMV replication takes place. More specifically, BMV replication protein 1a interacts with and recruits Cho2p (choline requiring 2), a host enzyme involved in PC synthesis, to the site of viral replication. These results suggest that PC synthesized at the site of VRC assembly, not the transport of existing PC, is responsible for the enhanced accumulation. Blocking PC synthesis by deleting the CHO2 gene resulted in VRCs with wider diameters than those in wild-type cells; however, BMV replication was significantly inhibited, highlighting the critical role of PC in VRC formation and viral replication. We further show that enhanced PC levels also accumulate at the replication sites of hepatitis C virus and poliovirus, revealing a conserved feature among a group of positive-strand RNA viruses. Our work also highlights a potential broad-spectrum antiviral strategy that would disrupt PC synthesis at the sites of viral replication but would not alter cellular processes.
Keywords: phospholipids; positive-strand RNA viruses; viral replication complexes; virus control; virus–host interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
An engineered mutant of a host phospholipid synthesis gene inhibits viral replication without compromising host fitness.J Biol Chem. 2019 Sep 20;294(38):13973-13982. doi: 10.1074/jbc.RA118.007051. Epub 2019 Jul 30. J Biol Chem. 2019. PMID: 31362985 Free PMC article.
-
An unrecognized function for COPII components in recruiting the viral replication protein BMV 1a to the perinuclear ER.J Cell Sci. 2016 Oct 1;129(19):3597-3608. doi: 10.1242/jcs.190082. Epub 2016 Aug 18. J Cell Sci. 2016. PMID: 27539921
-
Host Pah1p phosphatidate phosphatase limits viral replication by regulating phospholipid synthesis.PLoS Pathog. 2018 Apr 12;14(4):e1006988. doi: 10.1371/journal.ppat.1006988. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29649282 Free PMC article.
-
Bromovirus-induced remodeling of host membranes during viral RNA replication.Curr Opin Virol. 2014 Dec;9:104-10. doi: 10.1016/j.coviro.2014.09.018. Epub 2014 Oct 16. Curr Opin Virol. 2014. PMID: 25462441 Review.
-
Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication.Annu Rev Phytopathol. 2003;41:77-98. doi: 10.1146/annurev.phyto.41.052002.095717. Epub 2003 Mar 10. Annu Rev Phytopathol. 2003. PMID: 12651962 Review.
Cited by
-
A conserved viral amphipathic helix governs the replication site-specific membrane association.PLoS Pathog. 2022 Sep 1;18(9):e1010752. doi: 10.1371/journal.ppat.1010752. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36048900 Free PMC article.
-
A Single Point Mutation in the Rhinovirus 2B Protein Reduces the Requirement for Phosphatidylinositol 4-Kinase Class III Beta in Viral Replication.J Virol. 2018 Nov 12;92(23):e01462-18. doi: 10.1128/JVI.01462-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209171 Free PMC article.
-
Exploiting Connections for Viral Replication.Front Cell Dev Biol. 2021 Mar 18;9:640456. doi: 10.3389/fcell.2021.640456. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816489 Free PMC article.
-
Reconstitution of an RNA Virus Replicase in Artificial Giant Unilamellar Vesicles Supports Full Replication and Provides Protection for the Double-Stranded RNA Replication Intermediate.J Virol. 2020 Aug 31;94(18):e00267-20. doi: 10.1128/JVI.00267-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641477 Free PMC article.
-
Stearoly-CoA desaturase 1 differentiates early and advanced dengue virus infections and determines virus particle infectivity.PLoS Pathog. 2018 Aug 17;14(8):e1007261. doi: 10.1371/journal.ppat.1007261. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30118512 Free PMC article.
References
-
- Diaz A, Wang X. Bromovirus-induced remodeling of host membranes during viral RNA replication. Curr Opin Virol. 2014;9(12):104–110. - PubMed
-
- Wang X, Ahlquist P. Brome mosaic virus (Bromoviridae) In: Mahy BWJ, van Regenmortel MHV, editors. Encyclopedia of Virology. 3rd Ed. Elsevier; Oxford, UK: 2008. pp. 381–386.
-
- Kong F, Sivakumaran K, Kao C. The N-terminal half of the brome mosaic virus 1a protein has RNA capping-associated activities: Specificity for GTP and S-adenosylmethionine. Virology. 1999;259(1):200–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

