Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins
- PMID: 26858417
- PMCID: PMC4776536
- DOI: 10.1073/pnas.1516732113
Titin strain contributes to the Frank-Starling law of the heart by structural rearrangements of both thin- and thick-filament proteins
Abstract
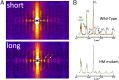
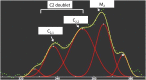
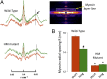
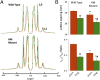
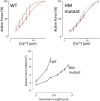
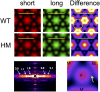
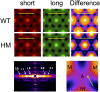
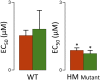
The Frank-Starling mechanism of the heart is due, in part, to modulation of myofilament Ca(2+) sensitivity by sarcomere length (SL) [length-dependent activation (LDA)]. The molecular mechanism(s) that underlie LDA are unknown. Recent evidence has implicated the giant protein titin in this cellular process, possibly by positioning the myosin head closer to actin. To clarify the role of titin strain in LDA, we isolated myocardium from either WT or homozygous mutant (HM) rats that express a giant splice isoform of titin, and subjected the muscles to stretch from 2.0 to 2.4 μm of SL. Upon stretch, HM compared with WT muscles displayed reduced passive force, twitch force, and myofilament LDA. Time-resolved small-angle X-ray diffraction measurements of WT twitching muscles during diastole revealed stretch-induced increases in the intensity of myosin (M2 and M6) and troponin (Tn3) reflections, as well as a reduction in cross-bridge radial spacing. Independent fluorescent probe analyses in relaxed permeabilized myocytes corroborated these findings. X-ray electron density reconstruction revealed increased mass/ordering in both thick and thin filaments. The SL-dependent changes in structure observed in WT myocardium were absent in HM myocardium. Overall, our results reveal a correlation between titin strain and the Frank-Starling mechanism. The molecular basis underlying this phenomenon appears not to involve interfilament spacing or movement of myosin toward actin but, rather, sarcomere stretch-induced simultaneous structural rearrangements within both thin and thick filaments that correlate with titin strain and myofilament LDA.
Keywords: fluorescent probes; myofilament length-dependent activation; passive force; rat; small-angle X-ray diffraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

