Seasonality in human cognitive brain responses
- PMID: 26858432
- PMCID: PMC4801294
- DOI: 10.1073/pnas.1518129113
Seasonality in human cognitive brain responses
Abstract
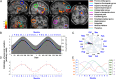
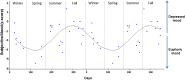
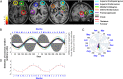
Daily variations in the environment have shaped life on Earth, with circadian cycles identified in most living organisms. Likewise, seasons correspond to annual environmental fluctuations to which organisms have adapted. However, little is known about seasonal variations in human brain physiology. We investigated annual rhythms of brain activity in a cross-sectional study of healthy young participants. They were maintained in an environment free of seasonal cues for 4.5 d, after which brain responses were assessed using functional magnetic resonance imaging (fMRI) while they performed two different cognitive tasks. Brain responses to both tasks varied significantly across seasons, but the phase of these annual rhythms was strikingly different, speaking for a complex impact of season on human brain function. For the sustained attention task, the maximum and minimum responses were located around summer and winter solstices, respectively, whereas for the working memory task, maximum and minimum responses were observed around autumn and spring equinoxes. These findings reveal previously unappreciated process-specific seasonality in human cognitive brain function that could contribute to intraindividual cognitive changes at specific times of year and changes in affective control in vulnerable populations.
Keywords: annual; attention; cognition; fMRI; season.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

