Membrane translocation of TRPC6 channels and endothelial migration are regulated by calmodulin and PI3 kinase activation
- PMID: 26858457
- PMCID: PMC4776520
- DOI: 10.1073/pnas.1600371113
Membrane translocation of TRPC6 channels and endothelial migration are regulated by calmodulin and PI3 kinase activation
Abstract
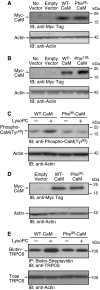
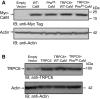
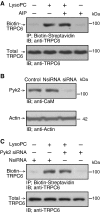
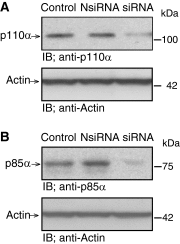
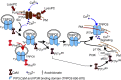
Lipid oxidation products, including lysophosphatidylcholine (lysoPC), activate canonical transient receptor potential 6 (TRPC6) channels leading to inhibition of endothelial cell (EC) migration in vitro and delayed EC healing of arterial injuries in vivo. The precise mechanism through which lysoPC activates TRPC6 channels is not known, but calmodulin (CaM) contributes to the regulation of TRPC channels. Using site-directed mutagenesis, cDNAs were generated in which Tyr(99) or Tyr(138) of CaM was replaced with Phe, generating mutant CaM, Phe(99)-CaM, or Phe(138)-CaM, respectively. In ECs transiently transfected with pcDNA3.1-myc-His-Phe(99)-CaM, but not in ECs transfected with pcDNA3.1-myc-His-Phe(138)-CaM, the lysoPC-induced TRPC6-CaM dissociation and TRPC6 externalization was disrupted. Also, the lysoPC-induced increase in intracellular calcium concentration was inhibited in ECs transiently transfected with pcDNA3.1-myc-His-Phe(99)-CaM. Blocking phosphorylation of CaM at Tyr(99) also reduced CaM association with the p85 subunit and subsequent activation of phosphatidylinositol 3-kinase (PI3K). This prevented the increase in phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and the translocation of TRPC6 to the cell membrane and reduced the inhibition of EC migration by lysoPC. These findings suggest that lysoPC induces CaM phosphorylation at Tyr(99) by a Src family kinase and that phosphorylated CaM activates PI3K to produce PIP3, which promotes TRPC6 translocation to the cell membrane.
Keywords: PI3 kinase; TRPC6; calmodulin; endothelial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Activation of the cytosolic calcium-independent phospholipase A2 β isoform contributes to TRPC6 externalization via release of arachidonic acid.J Biol Chem. 2021 Oct;297(4):101180. doi: 10.1016/j.jbc.2021.101180. Epub 2021 Sep 10. J Biol Chem. 2021. PMID: 34509476 Free PMC article.
-
Elucidation of a TRPC6-TRPC5 channel cascade that restricts endothelial cell movement.Mol Biol Cell. 2008 Aug;19(8):3203-11. doi: 10.1091/mbc.e07-08-0765. Epub 2008 May 21. Mol Biol Cell. 2008. PMID: 18495872 Free PMC article.
-
p85α regulatory subunit isoform controls PI3-kinase and TRPC6 membrane translocation.Cell Calcium. 2023 May;111:102718. doi: 10.1016/j.ceca.2023.102718. Epub 2023 Mar 15. Cell Calcium. 2023. PMID: 36934559 Free PMC article.
-
[Mechanism of cardiac hypertrophy via diacylglycerol-sensitive TRPC channels].Yakugaku Zasshi. 2010 Mar;130(3):295-302. doi: 10.1248/yakushi.130.295. Yakugaku Zasshi. 2010. PMID: 20190513 Review. Japanese.
-
[The role of calmodulin in calcium-dependent signalling in excitable cells].Postepy Biochem. 2012;58(4):393-402. Postepy Biochem. 2012. PMID: 23662433 Review. Polish.
Cited by
-
The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis.Int J Mol Sci. 2020 Jan 24;21(3):765. doi: 10.3390/ijms21030765. Int J Mol Sci. 2020. PMID: 31991573 Free PMC article. Review.
-
Inhibition of P110α and P110δ catalytic subunits of PI3 kinase reverses impaired arterial healing after injury in hypercholesterolemic male mice.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C943-C955. doi: 10.1152/ajpcell.00600.2020. Epub 2021 Mar 10. Am J Physiol Cell Physiol. 2021. PMID: 33689479 Free PMC article.
-
Munc13 mediates klotho-inhibitable diacylglycerol-stimulated exocytotic insertion of pre-docked TRPC6 vesicles.PLoS One. 2020 Mar 5;15(3):e0229799. doi: 10.1371/journal.pone.0229799. eCollection 2020. PLoS One. 2020. PMID: 32134975 Free PMC article.
-
Potential Mechanisms Underlying Inflammation-Enhanced Aminoglycoside-Induced Cochleotoxicity.Front Cell Neurosci. 2017 Nov 21;11:362. doi: 10.3389/fncel.2017.00362. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29209174 Free PMC article. Review.
-
A Putative Role for TRPC6 in Immune-Mediated Kidney Injury.Int J Mol Sci. 2023 Nov 16;24(22):16419. doi: 10.3390/ijms242216419. Int J Mol Sci. 2023. PMID: 38003608 Free PMC article. Review.
References
-
- Tran POT, Hinman LE, Unger GM, Sammak PJ. A wound-induced [Ca2+]i increase and its transcriptional activation of immediate early genes is important in the regulation of motility. Exp Cell Res. 1999;246(2):319–326. - PubMed
-
- Chaudhuri P, Colles SM, Damron DS, Graham LM. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arterioscler Thromb Vasc Biol. 2003;23(2):218–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

