High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates
- PMID: 26858459
- PMCID: PMC4776487
- DOI: 10.1073/pnas.1514018113
High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates
Abstract
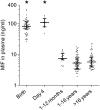
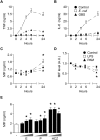
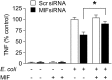
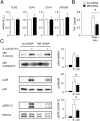
The vulnerability to infection of newborns is associated with a limited ability to mount efficient immune responses. High concentrations of adenosine and prostaglandins in the fetal and neonatal circulation hamper the antimicrobial responses of newborn immune cells. However, the existence of mechanisms counterbalancing neonatal immunosuppression has not been investigated. Remarkably, circulating levels of macrophage migration inhibitory factor (MIF), a proinflammatory immunoregulatory cytokine expressed constitutively, were 10-fold higher in newborns than in children and adults. Newborn monocytes expressed high levels of MIF and released MIF upon stimulation with Escherichia coli and group B Streptococcus, the leading pathogens of early-onset neonatal sepsis. Inhibition of MIF activity or MIF expression reduced microbial product-induced phosphorylation of p38 and ERK1/2 mitogen-activated protein kinases and secretion of cytokines. Recombinant MIF used at newborn, but not adult, concentrations counterregulated adenosine and prostaglandin E2-mediated inhibition of ERK1/2 activation and TNF production in newborn monocytes exposed to E. coli. In agreement with the concept that once infection is established high levels of MIF are detrimental to the host, treatment with a small molecule inhibitor of MIF reduced systemic inflammatory response, bacterial proliferation, and mortality of septic newborn mice. Altogether, these data provide a mechanistic explanation for how newborns may cope with an immunosuppressive environment to maintain a certain threshold of innate defenses. However, the same defense mechanisms may be at the expense of the host in conditions of severe infection, suggesting that MIF could represent a potential attractive target for immune-modulating adjunctive therapies for neonatal sepsis.
Keywords: Escherischia coli; adenosine; newborns; prostaglandin; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Macrophage migration inhibitory factor and host innate immune defenses against bacterial sepsis.J Infect Dis. 2003 Jun 15;187 Suppl 2:S385-90. doi: 10.1086/374752. J Infect Dis. 2003. PMID: 12792855
-
Macrophage migration inhibitory factor and innate immune responses to bacterial infections.Crit Care Med. 2001 Jul;29(7 Suppl):S13-5. doi: 10.1097/00003246-200107001-00006. Crit Care Med. 2001. PMID: 11445727 Review.
-
Macrophage migration inhibitory factor and host innate immune responses to microbes.Scand J Infect Dis. 2003;35(9):573-6. doi: 10.1080/00365540310016277. Scand J Infect Dis. 2003. PMID: 14620137 Review.
-
Macrophage migration inhibitory factor (MIF) modulates innate immune responses induced by endotoxin and Gram-negative bacteria.J Endotoxin Res. 2001;7(6):456-60. J Endotoxin Res. 2001. PMID: 11753217 Review.
-
Regulation of constitutive and microbial pathogen-induced human macrophage migration inhibitory factor (MIF) gene expression.Eur J Immunol. 2007 Dec;37(12):3509-21. doi: 10.1002/eji.200737357. Eur J Immunol. 2007. PMID: 18034423
Cited by
-
Key Enabling Technologies for Point-of-Care Diagnostics.Sensors (Basel). 2018 Oct 24;18(11):3607. doi: 10.3390/s18113607. Sensors (Basel). 2018. PMID: 30355989 Free PMC article. Review.
-
Trained Immunity Confers Broad-Spectrum Protection Against Bacterial Infections.J Infect Dis. 2020 Nov 9;222(11):1869-1881. doi: 10.1093/infdis/jiz692. J Infect Dis. 2020. PMID: 31889191 Free PMC article.
-
Modes of action and diagnostic value of miRNAs in sepsis.Front Immunol. 2022 Aug 5;13:951798. doi: 10.3389/fimmu.2022.951798. eCollection 2022. Front Immunol. 2022. PMID: 35990654 Free PMC article. Review.
-
The Potentials of Melatonin in the Prevention and Treatment of Bacterial Meningitis Disease.Molecules. 2021 Mar 5;26(5):1419. doi: 10.3390/molecules26051419. Molecules. 2021. PMID: 33808027 Free PMC article. Review.
-
The Multitasking Potential of Alarmins and Atypical Chemokines.Front Med (Lausanne). 2019 Jan 23;6:3. doi: 10.3389/fmed.2019.00003. eCollection 2019. Front Med (Lausanne). 2019. PMID: 30729111 Free PMC article. Review.
References
-
- Giannoni E, et al. Swiss Pediatric Sepsis Study Group Incidence and outcome of group B streptococcal sepsis in infants in Switzerland. Pediatr Infect Dis J. 2016;35(2):222–224. - PubMed
-
- Levy O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–390. - PubMed
-
- Aksoy E, et al. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109(7):2887–2893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

