Lipegfilgrastim in the management of chemotherapy-induced neutropenia of cancer patients
- PMID: 26858523
- PMCID: PMC4730998
- DOI: 10.2147/BTT.S58597
Lipegfilgrastim in the management of chemotherapy-induced neutropenia of cancer patients
Abstract
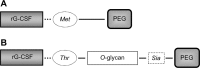
Neutropenia and febrile neutropenia (FN) are frequent and potentially fatal toxicities of myelosuppressive anticancer treatments. The introduction of granulocyte colony-stimulating factors (G-CSFs) in clinical practice has remarkably reduced the duration and severity of neutropenia, as well as the incidence of FN, thus allowing the administration of chemotherapeutic agents at the optimal dose and time with lower risk. The current scenario of G-CSFs in Europe includes filgrastim, lenograstim, some G-CSF biosimilars, and pegfilgrastim. Recently, a novel long-acting G-CSF, lipegfilgrastim, became available. Lipegfilgrastim is a glycopegylated G-CSF, alternative to pegfilgrastim, and has shown in randomized trials, to be equivalent to pegfilgrastim in reducing the incidence of severe neutropenia and FN in patients with breast cancer receiving chemotherapy, with a similar safety profile. Furthermore, lipegfilgrastim was more effective than the placebo in reducing the incidence of severe neutropenia, its duration, and time to absolute neutrophil count recovery, in patients with non-small cell lung cancer receiving myelosuppressive therapy. Although the number of studies currently published is still limited, lipegfilgrastim seems to be a promising drug in the management of chemotherapy-induced neutropenia.
Keywords: G-CSF; febrile neutropenia; granulocyte colony-stimulating factors; lipegfilgrastim; neutropenia; pegfilgrastim.
Figures
References
-
- Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. 2003;1(1):23–35. - PubMed
-
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. - PubMed
-
- de Naurois J, Novitzky-Basso I, Gill MJ, et al. ESMO Guidelines Working Group. Management of febrile neutropenia: ESMO clinical practice guidelines. Ann Oncol. 2010;21(Suppl 5):v252–v256. - PubMed
-
- Common Terminology Criteria for Adverse Advents v3.0 (CTCAE) [Internet] [Accessed August 13, 2015]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/....
-
- Nesher L, Rolston KV. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 2014;42(1):5–13. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

