Analysis of Expression Pattern and Genetic Deletion of Netrin5 in the Developing Mouse
- PMID: 26858598
- PMCID: PMC4726805
- DOI: 10.3389/fnmol.2016.00003
Analysis of Expression Pattern and Genetic Deletion of Netrin5 in the Developing Mouse
Abstract
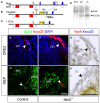
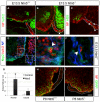
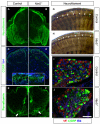
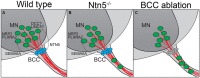
Boundary cap cells (BCC) are a transient, neural-crest-derived population found at the motor exit point (MEP) and dorsal root entry zone (DREZ) of the embryonic spinal cord. These cells contribute to the central/peripheral nervous system (CNS/PNS) boundary, and in their absence neurons and glia from the CNS migrate into the PNS. We found Netrin5 (Ntn5), a previously unstudied member of the netrin gene family, to be robustly expressed in BCC. We generated Ntn5 knockout mice and examined neurodevelopmental and BCC-related phenotypes. No abnormalities in cranial nerve guidance, dorsal root organization, or sensory projections were found. However, Ntn5 mutant embryos did have ectopic motor neurons (MNs) that migrated out of the ventral horn and into the motor roots. Previous studies have implicated semaphorin6A (Sema6A) in BCC signaling to plexinA2 (PlxnA2)/neuropilin2 (Nrp2) in MNs in restricting MN cell bodies to the ventral horn, particularly in the caudal spinal cord. In Ntn5 mutants, ectopic MNs are likely to be a different population, as more ectopias were found rostrally. Furthermore, ectopic MNs in Ntn5 mutants were not immunoreactive for NRP2. The netrin receptor deleted in colorectal cancer (DCC) is a potential receptor for NTN5 in MNs, as similar ectopic neurons were found in Dcc mutant mice, but not in mice deficient for other netrin receptors. Thus, Ntn5 is a novel netrin family member that is expressed in BCC, functioning to prevent MN migration out of the CNS.
Keywords: axon guidance; chemorepulsion; dorsal root entry zone; motor exit point.
Figures



Similar articles
-
Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system.Neural Dev. 2007 Dec 18;2:28. doi: 10.1186/1749-8104-2-28. Neural Dev. 2007. PMID: 18088409 Free PMC article.
-
Netrin 1 and Dcc signalling are required for confinement of central axons within the central nervous system.Development. 2014 Feb;141(3):594-603. doi: 10.1242/dev.099606. Development. 2014. PMID: 24449837
-
Commissural neurons transgress the CNS/PNS boundary in absence of ventricular zone-derived netrin 1.Development. 2018 Jan 17;145(2):dev159400. doi: 10.1242/dev.159400. Development. 2018. PMID: 29343638
-
[Boundary cap cells--a nest of neural stem cells in the peripheral nervous system].Bull Acad Natl Med. 2007 Oct;191(7):1383-92; discussion 1392-4. Bull Acad Natl Med. 2007. PMID: 18447060 Review. French.
-
Role for netrin-1 in sensory axonal guidance in higher vertebrates.Fukushima J Med Sci. 2009 Jun;55(1):1-6. doi: 10.5387/fms.55.1. Fukushima J Med Sci. 2009. PMID: 19999164 Review.
Cited by
-
Mutations in the netrin-1 gene cause congenital mirror movements.J Clin Invest. 2017 Nov 1;127(11):3923-3936. doi: 10.1172/JCI95442. Epub 2017 Sep 25. J Clin Invest. 2017. PMID: 28945198 Free PMC article.
-
The Slit-Robo signalling pathway in nervous system development: a comparative perspective from vertebrates and invertebrates.Open Biol. 2025 Jul;15(7):250026. doi: 10.1098/rsob.250026. Epub 2025 Jul 9. Open Biol. 2025. PMID: 40628293 Free PMC article. Review.
-
Recurrent DCC gene losses during bird evolution.Sci Rep. 2017 Feb 27;7:37569. doi: 10.1038/srep37569. Sci Rep. 2017. PMID: 28240285 Free PMC article.
-
Dorsal Root Injury-A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury.Cells. 2021 Aug 25;10(9):2185. doi: 10.3390/cells10092185. Cells. 2021. PMID: 34571835 Free PMC article. Review.
-
Motor Exit Point (MEP) Glia: Novel Myelinating Glia That Bridge CNS and PNS Myelin.Front Cell Neurosci. 2018 Oct 2;12:333. doi: 10.3389/fncel.2018.00333. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30356886 Free PMC article. Review.
References
-
- Achilli F., Bros-Facer V., Williams H. P., Banks G. T., Alqatari M., Chia R., et al. . (2009). An ENU-induced mutation in mouse glycyl-tRNA synthetase (GARS) causes peripheral sensory and motor phenotypes creating a model of Charcot-Marie-Tooth type 2D peripheral neuropathy. Dis. Model. Mech. 2, 359–373. 10.1242/dmm.002527 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

