Placental Responses to Changes in the Maternal Environment Determine Fetal Growth
- PMID: 26858656
- PMCID: PMC4731498
- DOI: 10.3389/fphys.2016.00012
Placental Responses to Changes in the Maternal Environment Determine Fetal Growth
Abstract
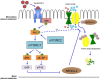
Placental responses to maternal perturbations are complex and remain poorly understood. Altered maternal environment during pregnancy such as hypoxia, stress, obesity, diabetes, toxins, altered nutrition, inflammation, and reduced utero-placental blood flow may influence fetal development, which can predispose to diseases later in life. The placenta being a metabolically active tissue responds to these perturbations by regulating the fetal supply of nutrients and oxygen and secretion of hormones into the maternal and fetal circulation. We have proposed that placental nutrient sensing integrates maternal and fetal nutritional cues with information from intrinsic nutrient sensing signaling pathways to balance fetal demand with the ability of the mother to support pregnancy by regulating maternal physiology, placental growth, and placental nutrient transport. Emerging evidence suggests that the nutrient-sensing signaling pathway mechanistic target of rapamycin (mTOR) plays a central role in this process. Thus, placental nutrient sensing plays a critical role in modulating maternal-fetal resource allocation, thereby affecting fetal growth and the life-long health of the fetus.
Keywords: fetal programming; maternal–fetal exchange; mechanistic target of rapamycin; placental nutrient sensing; pregnancy; syncytiotrophoblast.
Figures

References
-
- Anderson A. H., Fennessey P. V., Meschia G., Wilkening R. B., Battaglia F. C. (1997). Placental transport of threonine and its utilization in the normal and growth-restricted fetus. Am. J. Physiol. 272, E892–E900. - PubMed
-
- Armitage J. A., Khan I. Y., Taylor P. D., Nathanielsz P. W., Poston L. (2004). Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J. Physiol. 561, 355–377. 10.1113/jphysiol.2004.072009 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

