The "TSH Receptor Glo Assay" - A High-Throughput Detection System for Thyroid Stimulation
- PMID: 26858688
- PMCID: PMC4729884
- DOI: 10.3389/fendo.2016.00003
The "TSH Receptor Glo Assay" - A High-Throughput Detection System for Thyroid Stimulation
Abstract
Background: To identify novel small molecules against the TSH receptor, we developed a sensitive transcription-based luciferase high-throughput screening (HTS) system named the TSHR-Glo Assay (TSHR-Glo).
Methods: This assay uses double-transfected Chinese hamster ovary cells stably expressing the human TSHR and a cAMP-response element (CRE) construct fused to an improved luciferase reporter gene.
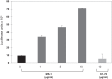
Results: The assay was highly responsive toward TSH in a dose-dependent manner with a TSH sensitivity of 10(-10)M (10 ± 1.12 μU/ml) and thyroid-stimulating antibodies, a hallmark of Graves' disease, could also be detected. The assay was validated against the standard indicator of HTS performance - the Z-factor (Z') - producing a score of 0.895. Using the TSHR-Glo assay, we screened 48,224 compounds from a diverse chemical library in duplicate plates at a fixed dose of 17 μM. Twenty molecules with the greatest activity out of 62 molecules that were identified by this technique were subsequently screened against the parent luciferase stable cell line in order to eliminate false positive stimulators.
Conclusion: Using this approach, we were able to identify specific agonists against the TSH receptor leading to the characterization of several TSH agonist molecules. Hence, the TSHR-Glo assay was a one-step cell-based HTS assay, which was successful in the discovery of novel small molecular agonists and for the detection of stimulating antibodies to the TSH receptor.
Keywords: TSH receptor; high-throughput; screening; small molecule; thyroid-stimulating hormone.
Figures





Similar articles
-
TSH Receptor Signaling Abrogation by a Novel Small Molecule.Front Endocrinol (Lausanne). 2016 Sep 27;7:130. doi: 10.3389/fendo.2016.00130. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27729899 Free PMC article.
-
A Gq Biased Small Molecule Active at the TSH Receptor.Front Endocrinol (Lausanne). 2020 Jun 26;11:372. doi: 10.3389/fendo.2020.00372. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32676053 Free PMC article.
-
New small molecule agonists to the thyrotropin receptor.Thyroid. 2015 Jan;25(1):51-62. doi: 10.1089/thy.2014.0119. Thyroid. 2015. PMID: 25333622 Free PMC article.
-
Bioassays for TSH Receptor Autoantibodies, from FRTL-5 Cells to TSH Receptor-LH/CG Receptor Chimeras: The Contribution of Leonard D. Kohn.Front Endocrinol (Lausanne). 2016 Jul 25;7:103. doi: 10.3389/fendo.2016.00103. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27504107 Free PMC article. Review.
-
Targeting thyroid diseases with TSH receptor analogs.Endocrinol Nutr. 2013 Dec;60(10):590-8. doi: 10.1016/j.endonu.2012.12.008. Epub 2013 Mar 26. Endocrinol Nutr. 2013. PMID: 23538281 Review.
Cited by
-
Cepharanthine blocks TSH receptor peptide presentation by HLA-DR3: Therapeutic implications to Graves' disease.J Autoimmun. 2020 Mar;108:102402. doi: 10.1016/j.jaut.2020.102402. Epub 2020 Jan 21. J Autoimmun. 2020. PMID: 31980336 Free PMC article.
-
Brief Report - Monoclonal Antibodies Illustrate the Difficulties in Measuring Blocking TSH Receptor Antibodies.Front Endocrinol (Lausanne). 2022 Jul 15;13:943459. doi: 10.3389/fendo.2022.943459. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35909553 Free PMC article.
-
Zebularine elevates STING expression and enhances cGAMP cancer immunotherapy in mice.Mol Ther. 2021 May 5;29(5):1758-1771. doi: 10.1016/j.ymthe.2021.02.005. Epub 2021 Feb 9. Mol Ther. 2021. PMID: 33571681 Free PMC article.
-
A Modifying Autoantigen in Graves' Disease.Endocrinology. 2019 May 1;160(5):1008-1020. doi: 10.1210/en.2018-01048. Endocrinology. 2019. PMID: 30822352 Free PMC article.
-
The Human TSHβ Subunit Proteins and Their Binding Sites on the TSH Receptor Using Molecular Dynamics Simulation.Endocrinology. 2020 Sep 1;161(9):bqaa125. doi: 10.1210/endocr/bqaa125. Endocrinology. 2020. PMID: 32738139 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

