Prospective Clinical Testing of Regulatory Dendritic Cells in Organ Transplantation
- PMID: 26858719
- PMCID: PMC4729892
- DOI: 10.3389/fimmu.2016.00015
Prospective Clinical Testing of Regulatory Dendritic Cells in Organ Transplantation
Abstract
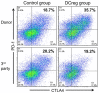
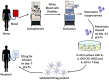
Dendritic cells (DC) are rare, professional antigen-presenting cells with ability to induce or regulate alloimmune responses. Regulatory DC (DCreg) with potential to down-modulate acute and chronic inflammatory conditions that occur in organ transplantation can be generated in vitro under a variety of conditions. Here, we provide a rationale for evaluation of DCreg therapy in clinical organ transplantation with the goal of promoting sustained, donor-specific hyporesponsiveness, while lowering the incidence and severity of rejection and reducing patients' dependence on anti-rejection drugs. Generation of donor- or recipient-derived DCreg that suppress T cell responses and prolong transplant survival in rodents or non-human primates has been well-described. Recently, good manufacturing practice (GMP)-grade DCreg have been produced at our Institution for prospective use in human organ transplantation. We briefly review experience of regulatory immune therapy in organ transplantation and describe our experience generating and characterizing human monocyte-derived DCreg. We propose a phase I/II safety study in which the influence of donor-derived DCreg combined with conventional immunosuppression on subclinical and clinical rejection and host alloimmune responses will be examined in detail.
Keywords: dendritic cells; immune regulation; renal transplantation.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

