Seed-Specific Expression of Spider Silk Protein Multimers Causes Long-Term Stability
- PMID: 26858734
- PMCID: PMC4729946
- DOI: 10.3389/fpls.2016.00006
Seed-Specific Expression of Spider Silk Protein Multimers Causes Long-Term Stability
Abstract
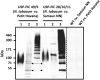
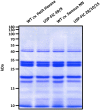
Seeds enable plants to germinate and to grow in situations of limited availability of nutrients. The stable storage of different seed proteins is a remarkable presumption for successful germination and growth. These strategies have been adapted and used in several molecular farming projects. In this study, we explore the benefits of seed-based expression to produce the high molecular weight spider silk protein FLAG using intein-based trans-splicing. Multimers larger than 460 kDa in size are routinely produced, which is above the native size of the FLAG protein. The storage of seeds for 8 weeks and 1 year at an ambient temperature of 15°C does not influence the accumulation level. Even the extended storage time does not influence the typical pattern of multimerized bands. These results show that seeds are the method of choice for stable accumulation of products of complex transgenes and have the capability for long-term storage at moderate conditions, an important feature for the development of suitable downstream processes.
Keywords: intein; protein trans-splicing; seed expression; spider silk proteins; tobacco.
Figures



Similar articles
-
Native-sized spider silk proteins synthesized in planta via intein-based multimerization.Transgenic Res. 2013 Apr;22(2):369-77. doi: 10.1007/s11248-012-9655-6. Epub 2012 Sep 22. Transgenic Res. 2013. PMID: 23001519
-
Transglutamination allows production and characterization of native-sized ELPylated spider silk proteins from transgenic plants.Plant Biotechnol J. 2014 Feb;12(2):265-75. doi: 10.1111/pbi.12135. Epub 2013 Nov 16. Plant Biotechnol J. 2014. PMID: 24237483
-
[Construction of spider silk functional platform via intein trans-splicing].Sheng Wu Gong Cheng Xue Bao. 2016 Dec 25;32(12):1704-1714. doi: 10.13345/j.cjb.160157. Sheng Wu Gong Cheng Xue Bao. 2016. PMID: 29034638 Chinese.
-
Spider silks from plants - a challenge to create native-sized spidroins.Biotechnol J. 2013 Oct;8(10):1183-92. doi: 10.1002/biot.201300204. Biotechnol J. 2013. PMID: 24092675 Review.
-
Seed-based expression systems for plant molecular farming.Plant Biotechnol J. 2010 Jun;8(5):588-606. doi: 10.1111/j.1467-7652.2010.00511.x. Plant Biotechnol J. 2010. PMID: 20500681 Review.
Cited by
-
Seeds as perfect factories for developing sustainable agriculture.Plant Reprod. 2018 Sep;31(3):201-202. doi: 10.1007/s00497-018-0340-7. Epub 2018 Jun 22. Plant Reprod. 2018. PMID: 29934741 No abstract available.
-
Genetic Containment for Molecular Farming.Plants (Basel). 2022 Sep 19;11(18):2436. doi: 10.3390/plants11182436. Plants (Basel). 2022. PMID: 36145835 Free PMC article. Review.
-
Seed- and leaf-based expression of FGF21-transferrin fusion proteins for oral delivery and treatment of non-alcoholic steatohepatitis.Front Plant Sci. 2022 Sep 29;13:998596. doi: 10.3389/fpls.2022.998596. eCollection 2022. Front Plant Sci. 2022. PMID: 36247628 Free PMC article.
-
Protein-Based Biological Materials: Molecular Design and Artificial Production.Chem Rev. 2023 Mar 8;123(5):2049-2111. doi: 10.1021/acs.chemrev.2c00621. Epub 2023 Jan 24. Chem Rev. 2023. PMID: 36692900 Free PMC article. Review.
-
Bioengineering of spider silks for the production of biomedical materials.Front Bioeng Biotechnol. 2022 Aug 9;10:958486. doi: 10.3389/fbioe.2022.958486. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36017345 Free PMC article. Review.
References
-
- Conrad U., Fiedler U., Artsaenko O., Phillips J. (1998). “Single-chain fv antibodies expressed in plants,” in Recombinant Proteins from Plants eds Cunningham C., Porter A. R. (New York City, NY: Humana Press; ) 103–127.
LinkOut - more resources
Full Text Sources
Other Literature Sources

