Molecular Evolution and Functional Diversification of Replication Protein A1 in Plants
- PMID: 26858742
- PMCID: PMC4731521
- DOI: 10.3389/fpls.2016.00033
Molecular Evolution and Functional Diversification of Replication Protein A1 in Plants
Abstract
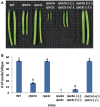
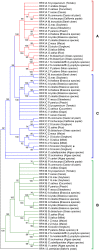
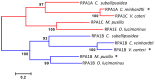
Replication protein A (RPA) is a heterotrimeric, single-stranded DNA binding complex required for eukaryotic DNA replication, repair, and recombination. RPA is composed of three subunits, RPA1, RPA2, and RPA3. In contrast to single RPA subunit genes generally found in animals and yeast, plants encode multiple paralogs of RPA subunits, suggesting subfunctionalization. Genetic analysis demonstrates that five Arabidopsis thaliana RPA1 paralogs (RPA1A to RPA1E) have unique and overlapping functions in DNA replication, repair, and meiosis. We hypothesize here that RPA1 subfunctionalities will be reflected in major structural and sequence differences among the paralogs. To address this, we analyzed amino acid and nucleotide sequences of RPA1 paralogs from 25 complete genomes representing a wide spectrum of plants and unicellular green algae. We find here that the plant RPA1 gene family is divided into three general groups termed RPA1A, RPA1B, and RPA1C, which likely arose from two progenitor groups in unicellular green algae. In the family Brassicaceae the RPA1B and RPA1C groups have further expanded to include two unique sub-functional paralogs RPA1D and RPA1E, respectively. In addition, RPA1 groups have unique domains, motifs, cis-elements, gene expression profiles, and pattern of conservation that are consistent with proposed functions in monocot and dicot species, including a novel C-terminal zinc-finger domain found only in plant RPA1C-like sequences. These results allow for improved prediction of RPA1 subunit functions in newly sequenced plant genomes, and potentially provide a unique molecular tool to improve classification of Brassicaceae species.
Keywords: DNA repair; RPA; meiosis; replication.
Figures






Similar articles
-
Genetic analysis of the Replication Protein A large subunit family in Arabidopsis reveals unique and overlapping roles in DNA repair, meiosis and DNA replication.Nucleic Acids Res. 2014 Mar;42(5):3104-18. doi: 10.1093/nar/gkt1292. Epub 2013 Dec 13. Nucleic Acids Res. 2014. PMID: 24335281 Free PMC article.
-
Functional Diversification of Replication Protein A Paralogs and Telomere Length Maintenance in Arabidopsis.Genetics. 2020 Aug;215(4):989-1002. doi: 10.1534/genetics.120.303222. Epub 2020 Jun 12. Genetics. 2020. PMID: 32532801 Free PMC article.
-
Replication protein A (RPA1a) is required for meiotic and somatic DNA repair but is dispensable for DNA replication and homologous recombination in rice.Plant Physiol. 2009 Dec;151(4):2162-73. doi: 10.1104/pp.109.142877. Epub 2009 Oct 7. Plant Physiol. 2009. PMID: 19812186 Free PMC article.
-
RPA homologs and ssDNA processing during meiotic recombination.Chromosoma. 2016 Jun;125(2):265-76. doi: 10.1007/s00412-015-0552-7. Epub 2015 Oct 31. Chromosoma. 2016. PMID: 26520106 Free PMC article. Review.
-
Replication protein A and more: single-stranded DNA-binding proteins in eukaryotic cells.Acta Biochim Biophys Sin (Shanghai). 2016 Jul;48(7):665-70. doi: 10.1093/abbs/gmw041. Epub 2016 May 4. Acta Biochim Biophys Sin (Shanghai). 2016. PMID: 27151292 Review.
Cited by
-
Antagonistic Effect of Sucrose Availability and Auxin on Rosa Axillary Bud Metabolism and Signaling, Based on the Transcriptomics and Metabolomics Analysis.Front Plant Sci. 2022 Mar 17;13:830840. doi: 10.3389/fpls.2022.830840. eCollection 2022. Front Plant Sci. 2022. PMID: 35392520 Free PMC article.
-
Suppression of plastid-to-nucleus gene transfer by DNA double-strand break repair.Nat Plants. 2025 Jun;11(6):1154-1164. doi: 10.1038/s41477-025-02005-w. Epub 2025 May 16. Nat Plants. 2025. PMID: 40379877 Free PMC article.
-
Evolution of Gene Duplication in Plants.Plant Physiol. 2016 Aug;171(4):2294-316. doi: 10.1104/pp.16.00523. Epub 2016 Jun 10. Plant Physiol. 2016. PMID: 27288366 Free PMC article. Review.
-
Increased Phosphorylation of Ser-Gln Sites on SUPPRESSOR OF GAMMA RESPONSE1 Strengthens the DNA Damage Response in Arabidopsis thaliana.Plant Cell. 2017 Dec;29(12):3255-3268. doi: 10.1105/tpc.17.00267. Epub 2017 Dec 5. Plant Cell. 2017. PMID: 29208704 Free PMC article.
-
Dynamic elements of replication protein A at the crossroads of DNA replication, recombination, and repair.Crit Rev Biochem Mol Biol. 2020 Oct;55(5):482-507. doi: 10.1080/10409238.2020.1813070. Epub 2020 Aug 28. Crit Rev Biochem Mol Biol. 2020. PMID: 32856505 Free PMC article. Review.
References
-
- Armstrong S. J., Franklin F. C., Jones G. H. (2001). Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana. J. Cell Sci. 114(Pt 23), 4207–4217. Available online at: http://jcs.biologists.org/content/114/23/4207.article-info - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

