A Quantitative Assessment of Factors Affecting the Technological Development and Adoption of Companion Diagnostics
- PMID: 26858745
- PMCID: PMC4730156
- DOI: 10.3389/fgene.2015.00357
A Quantitative Assessment of Factors Affecting the Technological Development and Adoption of Companion Diagnostics
Erratum in
-
Corrigendum: A Quantitative Assessment of Factors Affecting the Technological Development and Adoption of Companion Diagnostics.Front Genet. 2016 Jun 7;7:104. doi: 10.3389/fgene.2016.00104. eCollection 2016. Front Genet. 2016. PMID: 27375679 Free PMC article.
Abstract
Rapid innovation in (epi)genetics and biomarker sciences is driving a new drug development and product development pathway, with the personalized medicine era dominated by biologic therapeutics and companion diagnostics. Companion diagnostics (CDx) are tests and assays that detect biomarkers and specific mutations to elucidate disease pathways, stratify patient populations, and target drug therapies. CDx can substantially influence the development and regulatory approval for certain high-risk biologics. However, despite the increasingly important role of companion diagnostics in the realization of personalized medicine, in the USA, there are only 23 Food and Drug Administration (FDA) approved companion diagnostics on the market for 11 unique indications. Personalized medicines have great potential, yet their use is currently constrained. A major factor for this may lie in the increased complexity of the companion diagnostic and corresponding therapeutic development and adoption pathways. Understanding the market dynamics of companion diagnostic/therapeutic (CDx/Rx) pairs is important to further development and adoption of personalized medicine. Therefore, data collected on a variety of factors may highlight incentives or disincentives driving the development of companion diagnostics. Statistical analysis for 36 hypotheses resulted in two significant relationships and 34 non-significant relationships. The sensitivity of the companion diagnostic was the only factor that significantly correlated with the price of the companion diagnostic. This result indicates that while there is regulatory pressure for the diagnostic and pharmaceutical industry to collaborate and co-develop companion diagnostics for the approval of personalized therapeutics, there seems to be a lack of parallel economic collaboration to incentivize development of companion diagnostics.
Keywords: clinical adoption; combinational therapy; companion diagnostic; healthcare risk management; healthcare translation; personalized medicine; risk:benefit appraisal; stratified medicine.
Figures
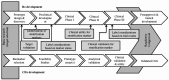



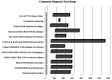

Similar articles
-
Perspective for the development of companion diagnostics and regulatory landscape to encourage personalized medicine in Japan.Breast Cancer. 2016 Jan;23(1):19-23. doi: 10.1007/s12282-015-0586-y. Epub 2015 Jan 22. Breast Cancer. 2016. PMID: 25605056
-
The Regulation of Companion Diagnostics: A Global Perspective.Ther Innov Regul Sci. 2013 Jul;47(4):405-415. doi: 10.1177/2168479013492734. Ther Innov Regul Sci. 2013. PMID: 30235516
-
Corrigendum: A Quantitative Assessment of Factors Affecting the Technological Development and Adoption of Companion Diagnostics.Front Genet. 2016 Jun 7;7:104. doi: 10.3389/fgene.2016.00104. eCollection 2016. Front Genet. 2016. PMID: 27375679 Free PMC article.
-
Companion diagnostics: a regulatory perspective from the last 5 years of molecular companion diagnostic approvals.Expert Rev Mol Diagn. 2015;15(7):869-80. doi: 10.1586/14737159.2015.1045490. Expert Rev Mol Diagn. 2015. PMID: 26109316 Review.
-
Overcoming regulatory and economic challenges facing pharmacogenomics.N Biotechnol. 2012 Sep 15;29(6):751-6. doi: 10.1016/j.nbt.2012.02.001. Epub 2012 Feb 19. N Biotechnol. 2012. PMID: 22370122 Review.
Cited by
-
Identification of genetic variants affecting reproduction traits in Vrindavani cattle.Mamm Genome. 2024 Mar;35(1):99-111. doi: 10.1007/s00335-023-10023-2. Epub 2023 Nov 4. Mamm Genome. 2024. PMID: 37924370
-
Applications of molecular testing in surgical pathology of the head and neck.Mod Pathol. 2017 Jan;30(s1):S104-S111. doi: 10.1038/modpathol.2016.192. Mod Pathol. 2017. PMID: 28060367 Review.
-
The "rights" of precision drug development for Alzheimer's disease.Alzheimers Res Ther. 2019 Aug 31;11(1):76. doi: 10.1186/s13195-019-0529-5. Alzheimers Res Ther. 2019. PMID: 31470905 Free PMC article. Review.
-
Pharmacogenomic phase transition from personalized medicine to patient-centric customized delivery.Pharmacogenomics J. 2020 Feb;20(1):1-18. doi: 10.1038/s41397-019-0135-8. Epub 2019 Dec 10. Pharmacogenomics J. 2020. PMID: 31819163 Review.
-
Sankey diagrams can clarify 'evidence attrition': A systematic review and meta-analysis of the effectiveness of rapid diagnostic tests for antimicrobial resistance.J Clin Epidemiol. 2022 Apr;144:173-184. doi: 10.1016/j.jclinepi.2021.11.032. Epub 2021 Nov 26. J Clin Epidemiol. 2022. PMID: 34843860 Free PMC article.
References
-
- Amir-Aslani A., Mangematin V. (2010). The future of drug discovery and development: shifting emphasis towards personalized medicine. Technol. Forecast. Soc. Change 77 203–217. 10.1016/j.techfore.2009.09.005 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

