The antiviral compound BIT225 inhibits HIV-1 replication in myeloid dendritic cells
- PMID: 26858771
- PMCID: PMC4745167
- DOI: 10.1186/s12981-016-0093-z
The antiviral compound BIT225 inhibits HIV-1 replication in myeloid dendritic cells
Abstract
Background: Previous studies with BIT225 (N-carbamimidoyl-5-(1-methyl-1H-pyrazol-4-yl)-2-naphthamide) have demonstrated a unique antiviral activity that blocks the release of HIV-1 from monocyte-derived macrophages (MDM). Antagonising the ion channel formed by HIV-1 Vpu, BIT225 preferentially targets de novo intracellular virus produced in 'virus-containing compartments' of MDM. In primary infections, dendritic cells (DC) are one of the first cells infected by HIV-1 and can transfer virus to more permissive CD4(+) T cells, making these cells an important target for novel antiviral therapies. To extend previous findings with BIT225, we aimed to further characterise the antiviral activity of BIT225 on HIV-1 replication in monocyte-derived DC (MDDC).
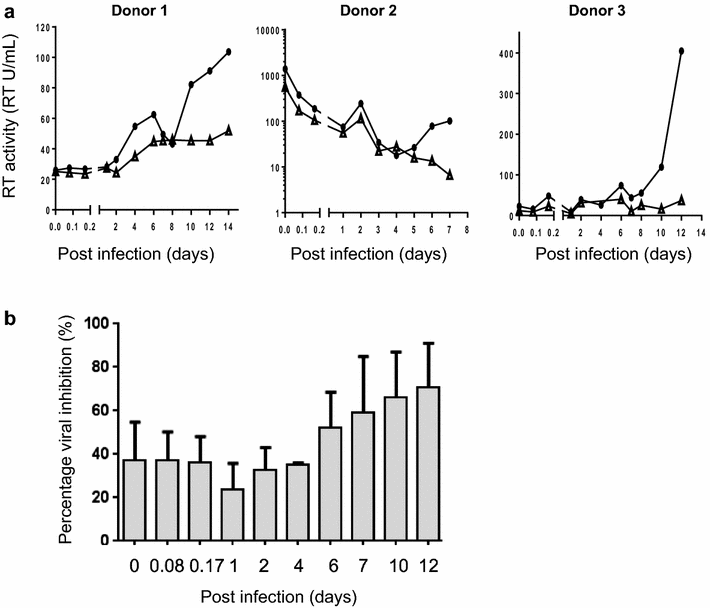
Results: The anti-HIV-1 activity of BIT225 was evaluated in vitro within MDDC alone and in co-cultures with activated CD4(+) T cells to examine the effect of the drug on HIV-1 transfer. Antiviral activity was determined by measuring HIV-1 reverse transcriptase activity in the culture supernatant of BIT225 treated and DMSO control cultures. A single dose of BIT225 resulted in a mean (SE) peak inhibition of HIV-1 release from MDDC by 74.5 % (±0.6) following 14 days of culture and a 6-fold reduction of HIV-1 transfer to activated uninfected CD4(+) T cells in co-culture.
Conclusions: HIV-1 release from MDDC was inhibited by BIT225. This data broadens the drug's antiviral activity profile within cells of the myeloid lineage. These findings suggest a potential role for BIT225 in reducing HIV-1 production and preventing viral dissemination in early and chronic infection and may assist in limiting virus spread with any ongoing viral replication during antiretroviral therapy.
Keywords: Antiviral; Dendritic cells; HIV-1; Myeloid; Viral transfer.
Figures

Similar articles
-
A Phase 1b/2a study of the safety, pharmacokinetics and antiviral activity of BIT225 in patients with HIV-1 infection.J Antimicrob Chemother. 2016 Mar;71(3):731-8. doi: 10.1093/jac/dkv389. Epub 2015 Nov 29. J Antimicrob Chemother. 2016. PMID: 26620101 Clinical Trial.
-
Antiviral efficacy of the novel compound BIT225 against HIV-1 release from human macrophages.Antimicrob Agents Chemother. 2010 Feb;54(2):835-45. doi: 10.1128/AAC.01308-09. Epub 2009 Dec 7. Antimicrob Agents Chemother. 2010. PMID: 19995924 Free PMC article.
-
Activity of reverse transcriptase inhibitors in monocyte-derived dendritic cells: a possible in vitro model for postexposure prophylaxis of sexual HIV transmission.AIDS Res Hum Retroviruses. 2002 Oct 10;18(15):1091-102. doi: 10.1089/088922202320567833. AIDS Res Hum Retroviruses. 2002. PMID: 12396448
-
Antiviral profile of HIV inhibitors in macrophages: implications for therapy.Curr Top Med Chem. 2004;4(9):1009-15. doi: 10.2174/1568026043388565. Curr Top Med Chem. 2004. PMID: 15134554 Review.
-
Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies.J Leukoc Biol. 2006 Nov;80(5):1103-10. doi: 10.1189/jlb.0606376. Epub 2006 Aug 24. J Leukoc Biol. 2006. PMID: 16931601 Review.
Cited by
-
Unravelling the Immunomodulatory Effects of Viral Ion Channels, towards the Treatment of Disease.Viruses. 2021 Oct 27;13(11):2165. doi: 10.3390/v13112165. Viruses. 2021. PMID: 34834972 Free PMC article. Review.
-
Post-infection treatment with the E protein inhibitor BIT225 reduces disease severity and increases survival of K18-hACE2 transgenic mice infected with a lethal dose of SARS-CoV-2.PLoS Pathog. 2023 Aug 7;19(8):e1011328. doi: 10.1371/journal.ppat.1011328. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37549173 Free PMC article.
-
Ion Channel Activity of Vpu Proteins Is Conserved throughout Evolution of HIV-1 and SIV.Viruses. 2016 Dec 1;8(12):325. doi: 10.3390/v8120325. Viruses. 2016. PMID: 27916968 Free PMC article.
-
Safety, Pharmacokinetics, and Antiviral Activity of a Novel HIV Antiviral, ABX464, in Treatment-Naive HIV-Infected Subjects in a Phase 2 Randomized, Controlled Study.Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00545-17. doi: 10.1128/AAC.00545-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28507108 Free PMC article. Clinical Trial.
References
-
- Wilkinson J, Ewart G, Luscombe C, McBride K, Ratanasuwan W, et al. A Phase 1b/2a study of the safety, pharmacokinetics and antiviral activity of BIT225 in patients with HIV-1 infection. J Antimicrob Chemother. 2015 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

