Human induced pluripotent stem cells derived endothelial cells mimicking vascular inflammatory response under flow
- PMID: 26858818
- PMCID: PMC4714980
- DOI: 10.1063/1.4940041
Human induced pluripotent stem cells derived endothelial cells mimicking vascular inflammatory response under flow
Abstract
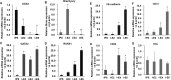
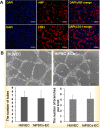
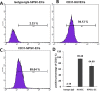
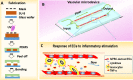
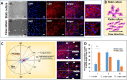
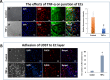
Endothelial cells (ECs) have great potential in vascular diseases research and regenerative medicine. Autologous human ECs are difficult to acquire in sufficient numbers in vitro, and human induced pluripotent stem cells (iPSCs) offer unique opportunity to generate ECs for these purposes. In this work, we present a new and efficient method to simply differentiate human iPSCs into functional ECs, which can respond to physiological level of flow and inflammatory stimulation on a fabricated microdevice. The endothelial-like cells were differentiated from human iPSCs within only one week, according to the inducing development principle. The expression of endothelial progenitor and endothelial marker genes (GATA2, RUNX1, CD34, and CD31) increased on the second and fourth days after the initial inducing process. The differentiated ECs exhibited strong expression of cells-specific markers (CD31 and von Willebrand factor antibody), similar to that present in human umbilical vein endothelial cells. In addition, the hiPSC derived ECs were able to form tubular structure and respond to vascular-like flow generated on a microdevice. Furthermore, the human induced pluripotent stem cell-endothelial cells (hiPSC-ECs) pretreated with tumor necrosis factor (TNF-α) were susceptible to adhesion to human monocyte line U937 under flow condition, indicating the feasibility of this hiPSCs derived microsystem for mimicking the inflammatory response of endothelial cells under physiological and pathological process.
Figures

Similar articles
-
High-efficient serum-free differentiation of endothelial cells from human iPS cells.Stem Cell Res Ther. 2022 Jun 11;13(1):251. doi: 10.1186/s13287-022-02924-x. Stem Cell Res Ther. 2022. PMID: 35690874 Free PMC article.
-
Human Induced Pluripotent Stem Cell-Derived Vascular Cells: Recent Progress and Future Directions.J Cardiovasc Dev Dis. 2021 Nov 4;8(11):148. doi: 10.3390/jcdd8110148. J Cardiovasc Dev Dis. 2021. PMID: 34821701 Free PMC article. Review.
-
Reprogramming of Adult Peripheral Blood Cells into Human Induced Pluripotent Stem Cells as a Safe and Accessible Source of Endothelial Cells.Stem Cells Dev. 2018 Jan 1;27(1):10-22. doi: 10.1089/scd.2017.0132. Epub 2017 Dec 11. Stem Cells Dev. 2018. PMID: 29117787 Free PMC article.
-
Generation of functional endothelial cells with progenitor-like features from murine induced pluripotent stem cells.Vascul Pharmacol. 2016 Nov;86:94-108. doi: 10.1016/j.vph.2016.07.008. Epub 2016 Aug 25. Vascul Pharmacol. 2016. PMID: 27568462
-
Induced Pluripotent Stem Cells as Vasculature Forming Entities.J Clin Med. 2019 Oct 25;8(11):1782. doi: 10.3390/jcm8111782. J Clin Med. 2019. PMID: 31731464 Free PMC article. Review.
Cited by
-
Sourcing cells for in vitro models of human vascular barriers of inflammation.Front Med Technol. 2022 Oct 21;4:979768. doi: 10.3389/fmedt.2022.979768. eCollection 2022. Front Med Technol. 2022. PMID: 36483299 Free PMC article. Review.
-
High-efficient serum-free differentiation of endothelial cells from human iPS cells.Stem Cell Res Ther. 2022 Jun 11;13(1):251. doi: 10.1186/s13287-022-02924-x. Stem Cell Res Ther. 2022. PMID: 35690874 Free PMC article.
-
iPSC-Derived Liver Organoids: A Journey from Drug Screening, to Disease Modeling, Arriving to Regenerative Medicine.Int J Mol Sci. 2020 Aug 27;21(17):6215. doi: 10.3390/ijms21176215. Int J Mol Sci. 2020. PMID: 32867371 Free PMC article. Review.
-
Human Induced Pluripotent Stem Cell-Derived Vascular Cells: Recent Progress and Future Directions.J Cardiovasc Dev Dis. 2021 Nov 4;8(11):148. doi: 10.3390/jcdd8110148. J Cardiovasc Dev Dis. 2021. PMID: 34821701 Free PMC article. Review.
-
iPSC-derived endothelial cell response to hypoxia via SDF1a/CXCR4 axis facilitates incorporation to revascularize ischemic retina.JCI Insight. 2020 Mar 26;5(6):e131828. doi: 10.1172/jci.insight.131828. JCI Insight. 2020. PMID: 32213707 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
