Three-Dimensional In Vitro Hepatic Constructs Formed Using Combinatorial Tapered Stencil for Cluster Culture (TASCL) Device
- PMID: 26858895
- PMCID: PMC4733838
- DOI: 10.3727/215517914X685187
Three-Dimensional In Vitro Hepatic Constructs Formed Using Combinatorial Tapered Stencil for Cluster Culture (TASCL) Device
Abstract
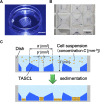
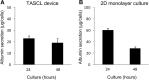
Attempts to create artificial liver tissue from various cells have been reported as an alternative method for liver transplantation and pharmaceutical testing. In the construction of artificial liver tissue, the selection of the cell source is the most important factor. However, if an appropriate environment (in vitro/in vivo) cannot be provided for various cells, it is not possible to obtain artificial liver tissue with the desired function. Therefore, we focused on the in vitro environment and produced liver tissues using MEMS technology. In the present study, we report a combinatorial TASCL device to prepare 3D cell constructs in vitro. The TASCL device was fabricated with an overall size of 10 mm × 10 mm with microwells and a top aperture (400 µm × 400 µm, 600 µm × 600 µm, 800 µm × 800 µm) and bottom aperture (40 µm × 40 µm, 80 µm × 80 µm, 160 µm × 160 µm) per microwell. The TASCL device can be easily installed on various culture dishes with tweezers. Using plastic dishes as the bottom surface of the combinatorial TASCL device, 3D hepatocyte constructs of uniform sizes (about ɸ 100 μm-ɸ 200 μm) were produced by increasing the seeding cell density of primary mouse hepatocytes. The 3D hepatocyte constructs obtained using the TASCL device were alive and secreted albumin. On the other hand, partially adhered primary mouse hepatocytes exhibited a cobblestone morphology on the collagen-coated bottom of the individual microwells using the combinatorial TASCL device. By changing the bottom substrate of the TASCL device, the culture environment of the cell constructs was easily changed to a 3D environment. The combinatorial TASCL device described in this report can be used quickly and simply. This device will be useful for preparing hepatocyte constructs for application in drug screening and cell medicine.
Keywords: Biomedical microdevices; Hepatic constructs; Primary hepatocytes; Tapered stencil for cluster culture (TASCL) device; Three-dimensional (3D) culture.
Figures



Similar articles
-
Enhanced Adipogenic Differentiation of Human Adipose-Derived Stem Cells in an In Vitro Microenvironment: The Preparation of Adipose-Like Microtissues Using a Three-Dimensional Culture.Cell Med. 2016 Sep 14;9(1-2):35-44. doi: 10.3727/215517916X693096. eCollection 2017 Jan 8. Cell Med. 2016. PMID: 28174673 Free PMC article.
-
Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device.Cell Med. 2015 Aug 26;8(1-2):47-56. doi: 10.3727/215517915X689056. eCollection 2015 Dec 17. Cell Med. 2015. PMID: 26858908 Free PMC article.
-
Embryonic body formation using the tapered soft stencil for cluster culture device.Biomaterials. 2011 May;32(15):3729-38. doi: 10.1016/j.biomaterials.2011.01.013. Epub 2011 Feb 26. Biomaterials. 2011. PMID: 21354615
-
New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures.Drug Metab Rev. 2003 May-Aug;35(2-3):145-213. doi: 10.1081/dmr-120023684. Drug Metab Rev. 2003. PMID: 12959414 Review.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Human placental hydrolysate promotes the long-term culture of hepatocyte-like cells derived from canine bone marrow.J Vet Med Sci. 2021 Jan 5;82(12):1821-1827. doi: 10.1292/jvms.20-0320. Epub 2020 Nov 2. J Vet Med Sci. 2021. PMID: 33132358 Free PMC article.
-
Cytokines in adipose-derived mesenchymal stem cells promote the healing of liver disease.World J Stem Cells. 2018 Nov 26;10(11):146-159. doi: 10.4252/wjsc.v10.i11.146. World J Stem Cells. 2018. PMID: 30631390 Free PMC article. Review.
-
Enhanced Adipogenic Differentiation of Human Adipose-Derived Stem Cells in an In Vitro Microenvironment: The Preparation of Adipose-Like Microtissues Using a Three-Dimensional Culture.Cell Med. 2016 Sep 14;9(1-2):35-44. doi: 10.3727/215517916X693096. eCollection 2017 Jan 8. Cell Med. 2016. PMID: 28174673 Free PMC article.
-
Long-Term Engineered Cultures of Primary Mouse Hepatocytes for Strain and Species Comparison Studies During Drug Development.Gene Expr. 2019 Nov 4;19(3):199-214. doi: 10.3727/105221619X15638857793317. Epub 2019 Jul 24. Gene Expr. 2019. PMID: 31340881 Free PMC article.
-
Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device.Cell Med. 2015 Aug 26;8(1-2):47-56. doi: 10.3727/215517915X689056. eCollection 2015 Dec 17. Cell Med. 2015. PMID: 26858908 Free PMC article.
References
-
- Basma H.; Soto-Gutiérrez A.; Yannam G. R.; Liu L.; Ito R.; Yamamoto T.; Ellis E.; Carson S. D.; Sato S.; Chen Y.; Muirhead D.; Navarro-Álvarez N.; Wong R. J.; Roy-Chowdhury J.; Platt J. L.; Mercer D. F.; Miller J. D.; Strom S. C.; Kobayashi N.; Fox I. J. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136:990–999; 2009. - PMC - PubMed
-
- Chen Y.; Soto-Gutiérrez A.; Navarro-Alvarez N.; Rivas-Carrillo J. D.; Yamatsuji T.; Shirakawa Y.; Tanaka N.; Basma H.; Fox I. J.; Kobayashi N. Instant hepatic differentiation of human embryonic stem cells using activin A and a deleted variant of HGF. Cell Transplant. 15:865–871; 2006. - PubMed
-
- Enosawa S.; Miyamoto Y.; Hirano A.; Suzuki S.; Kato N.; Yamada Y. Application of cell array 3D-culture system for cryopreserved human hepatocytes with low-attaching capability. Drug Metab. Rev. 39(Suppl. 1):342; 2007.
LinkOut - more resources
Full Text Sources

