Choice of Feeders Is Important When First Establishing iPSCs Derived From Primarily Cultured Human Deciduous Tooth Dental Pulp Cells
- PMID: 26858904
- PMCID: PMC4733907
- DOI: 10.3727/215517915X689038
Choice of Feeders Is Important When First Establishing iPSCs Derived From Primarily Cultured Human Deciduous Tooth Dental Pulp Cells
Abstract
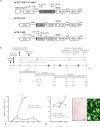
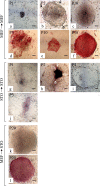
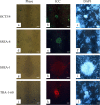
Feeder cells are generally required to maintain embryonic stem cells (ESCs)/induced pluripotent stem cells (iPSCs). Mouse embryonic fibroblasts (MEFs) isolated from fetuses and STO mouse stromal cell line are the most widely used feeder cells. The aim of this study was to determine which cells are suitable for establishing iPSCs from human deciduous tooth dental pulp cells (HDDPCs). Primary cultures of HDDPCs were cotransfected with three plasmids containing human OCT3/4, SOX2/KLF4, or LMYC/LIN28 and pmaxGFP by using a novel electroporation method, and then cultured in an ESC qualified medium for 15 days. Emerging colonies were reseeded onto mitomycin C-treated MEFs or STO cells. The colonies were serially passaged for up to 26 passages. During this period, colony morphology was assessed to determine whether cells exhibited ESC-like morphology and alkaline phosphatase activity to evaluate the state of cellular reprogramming. HDDPCs maintained on MEFs were successfully reprogrammed into iPSCs, whereas those maintained on STO cells were not. Once established, the iPSCs were maintained on STO cells without loss of pluripotency. Our results indicate that MEFs are better feeder cells than STO cells for establishing iPSCs. Feeder choice is a key factor enabling efficient generation of iPSCs.
Keywords: Deciduous tooth; Dental pulp; Feeder cell; Induced pluripotent stem cells (iPSCs); Mouse embryonic fibroblasts (MEFs); STO cells.
Figures



Similar articles
-
STO Feeder Cells Are Useful for Propagation of Primarily Cultured Human Deciduous Dental Pulp Cells by Eliminating Contaminating Bacteria and Promoting Cellular Outgrowth.Cell Med. 2013 Oct 25;6(1-2):75-81. doi: 10.3727/215517913X674234. eCollection 2013 Dec 30. Cell Med. 2013. PMID: 26858883 Free PMC article.
-
Gingival Fibroblasts as Autologous Feeders for Induced Pluripotent Stem Cells.J Dent Res. 2016 Jan;95(1):110-8. doi: 10.1177/0022034515611602. Epub 2015 Oct 14. J Dent Res. 2016. PMID: 26467419
-
Development of Reproducible and Scalable Culture Conditions for In Vitro Maintenance of Pig Embryonic Stem Cells Using the Sandoz Inbred Swiss Mouse Thioguanine-Resistant Ouabain-Resistant Cell Line as a Feeder Layer.Stem Cells Dev. 2023 Dec;32(23-24):747-757. doi: 10.1089/scd.2023.0171. Epub 2023 Oct 23. Stem Cells Dev. 2023. PMID: 37756363
-
Induced Tissue-Specific Stem Cells (iTSCs): Their Generation and Possible Use in Regenerative Medicine.Pharmaceutics. 2021 May 23;13(6):780. doi: 10.3390/pharmaceutics13060780. Pharmaceutics. 2021. PMID: 34071015 Free PMC article. Review.
-
Aging in the mouse and perspectives of rejuvenation through induced pluripotent stem cells (iPSCs).Results Probl Cell Differ. 2012;55:413-27. doi: 10.1007/978-3-642-30406-4_21. Results Probl Cell Differ. 2012. PMID: 22918818 Review.
Cited by
-
Stem Cells and Their Derivatives-Implications for Alveolar Bone Regeneration: A Comprehensive Review.Int J Mol Sci. 2021 Oct 29;22(21):11746. doi: 10.3390/ijms222111746. Int J Mol Sci. 2021. PMID: 34769175 Free PMC article. Review.
-
Increased Expression of Cell Surface SSEA-1 is Closely Associated with Naïve-Like Conversion from Human Deciduous Teeth Dental Pulp Cells-Derived iPS Cells.Int J Mol Sci. 2019 Apr 3;20(7):1651. doi: 10.3390/ijms20071651. Int J Mol Sci. 2019. PMID: 30987116 Free PMC article.
-
Sinking Our Teeth in Getting Dental Stem Cells to Clinics for Bone Regeneration.Int J Mol Sci. 2021 Jun 15;22(12):6387. doi: 10.3390/ijms22126387. Int J Mol Sci. 2021. PMID: 34203719 Free PMC article. Review.
-
Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability.Sci Rep. 2019 Feb 6;9(1):1490. doi: 10.1038/s41598-018-37291-2. Sci Rep. 2019. PMID: 30728386 Free PMC article.
-
The Role of Genetically Modified Human Feeder Cells in Maintaining the Integrity of Primary Cultured Human Deciduous Dental Pulp Cells.J Clin Med. 2022 Oct 15;11(20):6087. doi: 10.3390/jcm11206087. J Clin Med. 2022. PMID: 36294410 Free PMC article.
References
-
- Amit M.; Margulets V.; Segev H.; Shariki K.; Laevsky I.; Coleman R.; Itskovitz-Eldor J. Human feeder layers for human embryonic stem cells. Biol. Reprod. 68(6):2150–2156; 2003. - PubMed
-
- Beattie G. M.; Lopez A. D.; Bucay N.; Hinton A.; Firpo M. T.; King C. C.; Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 23(4):489–495; 2005. - PubMed
-
- Chen M.; Sun X.; Jiang R.; Shen W.; Zhong X.; Liu B.; Qi Y.; Huang B.; Xiang A. P.; Ge J. Role of MEF feeder cells in direct reprogramming of mousetail-tip fibroblasts. Cell Biol. Int. 33(12):1268–1273; 2009. - PubMed
-
- Eiselleova L.; Peterkova I.; Neradil J.; Slaninova I.; Hampl A.; Dvorak P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int. J. Dev. Biol. 52(4):353–363; 2008. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

