Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device
- PMID: 26858908
- PMCID: PMC4733911
- DOI: 10.3727/215517915X689056
Spheroid Formation and Evaluation of Hepatic Cells in a Three-Dimensional Culture Device
Abstract
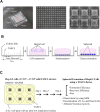
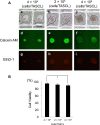
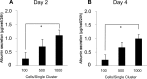
In drug discovery, it is very important to evaluate liver cells within an organism. Compared to 2D culture methods, the development of 3D culture techniques for liver cells has been successful in maintaining long-term liver functionality with the formation of a hepatic-specific structure. The key to performing drug testing is the establishment of a stable in vitro evaluation system. In this article, we report a Tapered Stencil for Cluster Culture (TASCL) device developed to create liver spheroids in vitro. The TASCL device will be applied as a toxicity evaluation system for drug discovery. The TASCL device was created with an overall size of 10 mm × 10 mm, containing 400 microwells with a top aperture (500 µm × 500 µm) and a bottom aperture (300 µm diameter circular) per microwell. We evaluated the formation, recovery, and size of HepG2 spheroids in the TASCL device. The formation and recovery were both nearly 100%, and the size of the HepG2 spheroids increased with an increase in the initial cell seeding density. There were no significant differences in the sizes of the spheroids among the microwells. In addition, the HepG2 spheroids obtained using the TASCL device were alive and produced albumin. The morphology of the HepG2 spheroids was investigated using FE-SEM. The spheroids in the microwells exhibited perfectly spherical aggregation. In this report, by adjusting the size of the microwells of the TASCL device, uniform HepG2 spheroids were created, and the device facilitated more precise measurements of the liver function per HepG2 spheroid. Our TASCL device will be useful for application as a toxicity evaluation system for drug testing.
Keywords: Human hepatocellular carcinoma (HepG2) cells; Medical evaluation; Spheroid; Tapered stencil for cluster culture (TASCL); Three-dimensional (3D) culture.
Figures



Similar articles
-
Three-Dimensional In Vitro Hepatic Constructs Formed Using Combinatorial Tapered Stencil for Cluster Culture (TASCL) Device.Cell Med. 2014 Dec 12;7(2):67-74. doi: 10.3727/215517914X685187. eCollection 2015 Feb 8. Cell Med. 2014. PMID: 26858895 Free PMC article.
-
Enhanced Adipogenic Differentiation of Human Adipose-Derived Stem Cells in an In Vitro Microenvironment: The Preparation of Adipose-Like Microtissues Using a Three-Dimensional Culture.Cell Med. 2016 Sep 14;9(1-2):35-44. doi: 10.3727/215517916X693096. eCollection 2017 Jan 8. Cell Med. 2016. PMID: 28174673 Free PMC article.
-
Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids.Lab Chip. 2018 Aug 21;18(17):2547-2562. doi: 10.1039/c8lc00333e. Lab Chip. 2018. PMID: 30019731
-
Optimization of Albumin Secretion and Metabolic Activity of Cytochrome P450 1A1 of Human Hepatoblastoma HepG2 Cells in Multicellular Spheroids by Controlling Spheroid Size.Biol Pharm Bull. 2017;40(3):334-338. doi: 10.1248/bpb.b16-00833. Biol Pharm Bull. 2017. PMID: 28250275
-
Micropatterned culture of HepG2 spheroids using microwell chip with honeycomb-patterned polymer film.J Biosci Bioeng. 2014 Oct;118(4):455-60. doi: 10.1016/j.jbiosc.2014.03.006. Epub 2014 Apr 16. J Biosci Bioeng. 2014. PMID: 24742630
Cited by
-
Fabrication Method for Shape-Controlled 3D Tissue Using High-Porosity Porous Structure.Bioengineering (Basel). 2024 Feb 5;11(2):160. doi: 10.3390/bioengineering11020160. Bioengineering (Basel). 2024. PMID: 38391646 Free PMC article.
-
Functional Evaluation of 3D Liver Models Labeled with Polysaccharide Functionalized Magnetic Nanoparticles.Materials (Basel). 2022 Nov 5;15(21):7823. doi: 10.3390/ma15217823. Materials (Basel). 2022. PMID: 36363415 Free PMC article.
-
High-glucose 3D INS-1 cell model combined with a microfluidic circular concentration gradient generator for high throughput screening of drugs against type 2 diabetes.RSC Adv. 2018 Jul 16;8(45):25409-25416. doi: 10.1039/c8ra04040k. eCollection 2018 Jul 16. RSC Adv. 2018. PMID: 35539797 Free PMC article.
-
Development of a tunable method to generate various three-dimensional microstructures by replenishing macromolecules such as extracellular matrix components and polysaccharides.Sci Rep. 2020 Apr 16;10(1):6567. doi: 10.1038/s41598-020-63621-4. Sci Rep. 2020. PMID: 32300241 Free PMC article.
-
The Microwell-mesh: A high-throughput 3D prostate cancer spheroid and drug-testing platform.Sci Rep. 2018 Jan 10;8(1):253. doi: 10.1038/s41598-017-18050-1. Sci Rep. 2018. PMID: 29321576 Free PMC article.
References
-
- Basma H.; Soto-Gutiérrez A.; Yannam G. R.; Liu L.; Ito R.; Yamamoto T.; Ellis E.; Carson S. D.; Sato S.; Chen Y.; Muirhead D.; Navarro-Álvarez N.; Wong R. J.; Roy-Chowdhury J.; Platt J. L.; Mercer D. F.; Miller J. D.; Strom S. C.; Kobayashi N.; Fox I. J. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136:990–999; 2009. - PMC - PubMed
-
- Chen Y.; Soto-Gutiérrez A.; Navarro-Alvarez N.; Rivas-Carrillo J. D.; Yamatsuji T.; Shirakawa Y.; Tanaka N.; Basma H.; Fox I. J.; Kobayashi N. Instant hepatic differentiation of human embryonic stem cells using activin A and a deleted variant of HGF. Cell Transplant. 15:865–871; 2006. - PubMed
-
- Enosawa S.; Miyamoto Y.; Hirano A.; Suzuki S.; Kato N.; Yamada Y. Application of cell array 3D-culture system for cryopreserved human hepatocytes with low-attaching capability. Drug Metab. Rev. 39(Suppl. 1):342; 2007.
-
- Fahmi O. A.; Kish M.; Boldt S.; Obach R. S. Cytochrome P450 3A4 mRNA is a more reliable marker than CYP3A4 activity for detecting pregnane X receptor-activated induction of drug-metabolizing enzymes. Drug Metab. Dispos. 38(9):1605–1611; 2010. - PubMed
LinkOut - more resources
Full Text Sources

