Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum
- PMID: 26858947
- PMCID: PMC4729952
- DOI: 10.3389/fcell.2015.00083
Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum
Abstract
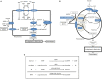
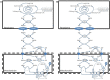
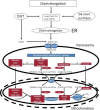
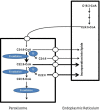
Peroxisomes are unique subcellular organelles which play an indispensable role in several key metabolic pathways which include: (1.) etherphospholipid biosynthesis; (2.) fatty acid beta-oxidation; (3.) bile acid synthesis; (4.) docosahexaenoic acid (DHA) synthesis; (5.) fatty acid alpha-oxidation; (6.) glyoxylate metabolism; (7.) amino acid degradation, and (8.) ROS/RNS metabolism. The importance of peroxisomes for human health and development is exemplified by the existence of a large number of inborn errors of peroxisome metabolism in which there is an impairment in one or more of the metabolic functions of peroxisomes. Although the clinical signs and symptoms of affected patients differ depending upon the enzyme which is deficient and the extent of the deficiency, the disorders involved are usually (very) severe diseases with neurological dysfunction and early death in many of them. With respect to the role of peroxisomes in metabolism it is clear that peroxisomes are dependent on the functional interplay with other subcellular organelles to sustain their role in metabolism. Indeed, whereas mitochondria can oxidize fatty acids all the way to CO2 and H2O, peroxisomes are only able to chain-shorten fatty acids and the end products of peroxisomal beta-oxidation need to be shuttled to mitochondria for full oxidation to CO2 and H2O. Furthermore, NADH is generated during beta-oxidation in peroxisomes and beta-oxidation can only continue if peroxisomes are equipped with a mechanism to reoxidize NADH back to NAD(+), which is now known to be mediated by specific NAD(H)-redox shuttles. In this paper we describe the current state of knowledge about the functional interplay between peroxisomes and other subcellular compartments notably the mitochondria and endoplasmic reticulum for each of the metabolic pathways in which peroxisomes are involved.
Keywords: endoplasmic reticulum; etherphospholipids; fatty acids; genetic diseases; metabolism; mitochondria; peroxisomal diseases; peroxisomes.
Figures




References
-
- Amery L., Fransen M., De Nys K., Mannaerts G. P., Van Veldhoven P. P. (2000) Mitochondrial peroxisomal targeting of 2-methylacyl-CoA racemase in humans. J. Lipid Res. 41, 1752–1759. - PubMed
-
- Antonenkov V. D., Van Veldhoven P. P., Waelkens E., Mannaerts G. P. (1997). Substrate specificities of 3-oxoacyl-CoA thiolase A and sterol carrier protein 2/3-oxoacyl-CoA thiolase purified from normal rat liver peroxisomes. Sterol carrier protein 2/3-oxoacyl-CoA thiolase is involved in the metabolism of 2-methyl-branched fatty acids and bile acid intermediates. J. Biol. Chem. 272, 26023–26031. 10.1074/jbc.272.41.26023 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

