Manufacturing of AcMNPV baculovirus vectors to enable gene therapy trials
- PMID: 26858963
- PMCID: PMC4729316
- DOI: 10.1038/mtm.2015.50
Manufacturing of AcMNPV baculovirus vectors to enable gene therapy trials
Abstract
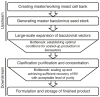
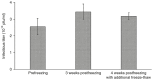
Over the past two decades, baculoviruses have become workhorse research tools for transient transgene expression. Although they have not yet been used directly as a gene therapy vector in the clinical setting, numerous preclinical studies have suggested the highly promising potential of baculovirus as a delivery vector for a variety of therapeutic applications including vaccination, tissue engineering, and cancer treatment. As such, there is growing interest in using baculoviruses as human gene therapy vectors, which has led to advances in baculovirus bioprocessing methods. This review provides an overview of the current approaches for scaled-up amplification, concentration, purification, and formulation of AcMNPV baculoviruses, and highlights the key regulatory requirements that must be met before gene therapy clinical trials can be initiated.
Figures
Similar articles
-
The Major Hurdle for Effective Baculovirus Transduction into Mammalian Cells Is Passing Early Endosomes.J Virol. 2019 Jul 17;93(15):e00709-19. doi: 10.1128/JVI.00709-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092570 Free PMC article.
-
Requirements for baculoviruses for clinical gene therapy applications.J Invertebr Pathol. 2011 Jul;107 Suppl:S106-12. doi: 10.1016/j.jip.2011.05.010. J Invertebr Pathol. 2011. PMID: 21784225 Review.
-
Solutions against emerging infectious and noninfectious human diseases through the application of baculovirus technologies.Appl Microbiol Biotechnol. 2021 Nov;105(21-22):8195-8226. doi: 10.1007/s00253-021-11615-1. Epub 2021 Oct 7. Appl Microbiol Biotechnol. 2021. PMID: 34618205 Free PMC article. Review.
-
Bioprocessing of baculovirus vectors: a review.Curr Gene Ther. 2010 Jun;10(3):174-86. doi: 10.2174/156652310791321288. Curr Gene Ther. 2010. PMID: 20380645 Review.
-
Advancements in molecular design and bioprocessing of recombinant adeno-associated virus gene delivery vectors using the insect-cell baculovirus expression platform.Biotechnol J. 2021 Apr;16(4):e2000021. doi: 10.1002/biot.202000021. Epub 2021 Jan 25. Biotechnol J. 2021. PMID: 33277815 Review.
Cited by
-
Mixtures of Insect-Pathogenic Viruses in a Single Virion: towards the Development of Custom-Designed Insecticides.Appl Environ Microbiol. 2021 Jan 15;87(3):e02180-20. doi: 10.1128/AEM.02180-20. Print 2021 Jan 15. Appl Environ Microbiol. 2021. PMID: 33187994 Free PMC article.
-
Current status and development prospects of aquatic vaccines.Front Immunol. 2022 Nov 10;13:1040336. doi: 10.3389/fimmu.2022.1040336. eCollection 2022. Front Immunol. 2022. PMID: 36439092 Free PMC article. Review.
-
In vivo genome editing in animals using AAV-CRISPR system: applications to translational research of human disease.F1000Res. 2017 Dec 20;6:2153. doi: 10.12688/f1000research.11243.1. eCollection 2017. F1000Res. 2017. PMID: 29333255 Free PMC article. Review.
-
Targeted Inhibition of the miR-199a/214 Cluster by CRISPR Interference Augments the Tumor Tropism of Human Induced Pluripotent Stem Cell-Derived Neural Stem Cells under Hypoxic Condition.Stem Cells Int. 2016;2016:3598542. doi: 10.1155/2016/3598542. Epub 2016 Nov 14. Stem Cells Int. 2016. PMID: 27965712 Free PMC article.
-
Baculovirus-assisted Reovirus Infection in Monolayer and Spheroid Cultures of Glioma cells.Sci Rep. 2017 Dec 15;7(1):17654. doi: 10.1038/s41598-017-17709-z. Sci Rep. 2017. PMID: 29247249 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

