Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in CML Study IV
- PMID: 26859076
- PMCID: PMC4895174
- DOI: 10.1038/leu.2016.20
Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in CML Study IV
Abstract
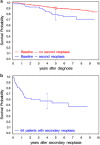
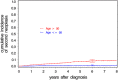
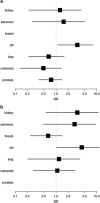
Treatment of chronic myeloid leukemia (CML) has been profoundly improved by the introduction of tyrosine kinase inhibitors (TKIs). Long-term survival with imatinib is excellent with a 8-year survival rate of ∼88%. Long-term toxicity of TKI treatment, especially carcinogenicity, has become a concern. We analyzed data of the CML study IV for the development of secondary malignancies. In total, 67 secondary malignancies were found in 64 of 1525 CML patients in chronic phase treated with TKI (n=61) and interferon-α only (n=3). The most common malignancies (n⩾4) were prostate, colorectal and lung cancer, non-Hodgkin's lymphoma (NHL), malignant melanoma, non-melanoma skin tumors and breast cancer. The standardized incidence ratio (SIR) for all malignancies excluding non-melanoma skin tumors was 0.88 (95% confidence interval (0.63-1.20)) for men and 1.06 (95% CI 0.69-1.55) for women. SIRs were between 0.49 (95% CI 0.13-1.34) for colorectal cancer in men and 4.29 (95% CI 1.09-11.66) for NHL in women. The SIR for NHL was significantly increased for men and women. An increase in the incidence of secondary malignancies could not be ascertained. The increased SIR for NHL has to be considered and long-term follow-up of CML patients is warranted, as the rate of secondary malignancies may increase over time.
Conflict of interest statement
BH has received honoraria from Bristol-Myers Squibb (BMS) and research funding from Novartis; SWK honoraria and research funding by Novartis; MCM honoraria and research funding from Novartis, BMS, ARIAD and Pfizer; MP honoraria from BMS and consultancy from Novartis; AH honoraria from Novartis, BMS, ARIAD, consultancy from Novartis and research funding from Novartis, ARIAD and Pfizer; RH research funding from Novartis and BMS and SS honoraria from Novartis, BMS, Pfizer, ARIAD and research funding from Novartis and BMS. The other authors declare no conflict of interest.
Figures
References
-
- Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol 2011; 29: 1634–1642. - PubMed
-
- Hehlmann R, Muller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol 2014; 32: 415–423. - PubMed
-
- Gambacorti-Passerini C, Antolini L, Mahon FX, Guilhot F, Deininger M, Fava C et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst 2011; 103: 553–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

