Neurogenic Effects of Cell-Free Extracts of Adipose Stem Cells
- PMID: 26859291
- PMCID: PMC4747593
- DOI: 10.1371/journal.pone.0148691
Neurogenic Effects of Cell-Free Extracts of Adipose Stem Cells
Abstract
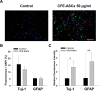
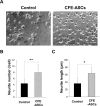
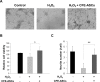
Stem-cell-based therapies are regarded as promising treatments for neurological disorders, and adipose-derived stem cells (ASCs) are a feasible source of clinical application of stem cell. Recent studies have shown that stem cells have a therapeutic potential for use in the treatment of various illnesses through paracrine action. To examine the effects of cell components of ASCs on neural stem cells (NSCs), we treated cell-free extracts of ASCs (CFE-ASCs) containing various components with brain-derived NSCs. To elucidate the effects of CFE-ASCs in NSC proliferation, we treated mouse subventricular zone-derived cultured NSCs with various doses of CFE-ASCs. As a result, CFE-ASCs were found to induce the proliferation of NSCs under conditions of growth factor deprivation in a dose-dependent manner (p<0.01). CFE-ASCs increase the expression of neuron and astrocyte differentiation markers including Tuj-1 (p<0.05) and glial fibrillary acidic protein (p<0.01) without altering the cell's fate in differentiating NSCs. In addition, treatment with CFE-ASCs induces an increase in neurite numbers (p<0.01) and lengths of NSCs (p<0.05). Furthermore, CFE-ASCs rescue the hydrogen peroxide-induced reduction of NSCs' viability (p<0.05) and neurite branching (p<0.01). Findings from our study indicate that CFE-ASCs support the survival, proliferation and differentiation of NSCs accompanied with neurite outgrowth, suggesting that CFE-ASCs can modulate neurogenesis in the central nervous system.
Conflict of interest statement
Figures

Similar articles
-
Neurotrophin-3 promotes proliferation and cholinergic neuronal differentiation of bone marrow- derived neural stem cells via notch signaling pathway.Life Sci. 2016 Dec 1;166:131-138. doi: 10.1016/j.lfs.2016.10.004. Epub 2016 Oct 6. Life Sci. 2016. PMID: 27720999
-
Seven days post-injury fate and effects of genetically labelled adipose-derived mesenchymal cells on a rat traumatic brain injury experimental model.Histol Histopathol. 2017 Oct;32(10):1041-1055. doi: 10.14670/HH-11-864. Epub 2016 Dec 30. Histol Histopathol. 2017. PMID: 28035654
-
Ventral midbrain neural stem cells have delayed neurogenic potential in vitro.Neurosci Lett. 2014 Jan 24;559:193-8. doi: 10.1016/j.neulet.2013.12.009. Epub 2013 Dec 14. Neurosci Lett. 2014. PMID: 24342440
-
Neural stem cells, neural progenitors, and neurotrophic factors.Cell Transplant. 2007;16(2):133-50. Cell Transplant. 2007. PMID: 17474295 Review.
-
Olfactory ensheathing cells promote differentiation of neural stem cells and robust neurite extension.Stem Cell Rev Rep. 2014 Dec;10(6):772-85. doi: 10.1007/s12015-014-9539-7. Stem Cell Rev Rep. 2014. PMID: 24996386 Free PMC article. Review.
Cited by
-
Adult stem cell response to doped bioactive borate glass.J Mater Sci Mater Med. 2020 Jan 21;31(2):13. doi: 10.1007/s10856-019-6353-4. J Mater Sci Mater Med. 2020. PMID: 31965357
-
Cytosolic Extract of Human Adipose Stem Cells Reverses the Amyloid Beta-Induced Mitochondrial Apoptosis via P53/Foxo3a Pathway.PLoS One. 2017 Jan 3;12(1):e0168859. doi: 10.1371/journal.pone.0168859. eCollection 2017. PLoS One. 2017. PMID: 28046000 Free PMC article.
-
Intravenous Administration of Adipose-Derived Stem Cell Protein Extracts Improves Neurological Deficits in a Rat Model of Stroke.Stem Cells Int. 2017;2017:2153629. doi: 10.1155/2017/2153629. Epub 2017 Feb 7. Stem Cells Int. 2017. PMID: 28265288 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

