Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry
- PMID: 26859324
- PMCID: PMC4775376
- DOI: 10.1021/acs.biochem.5b01378
Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry
Abstract
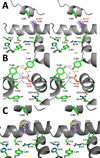
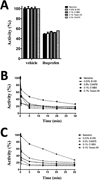
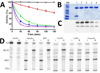
Aspirin and other nonsteroidal anti-inflammatory drugs target the cyclooxygenase enzymes (COX-1 and COX-2) to block the formation of prostaglandins. Aspirin is unique in that it covalently modifies each enzyme by acetylating Ser-530 within the cyclooxygenase active site. Acetylation of COX-1 leads to complete loss of activity, while acetylation of COX-2 results in the generation of the monooxygenated product 15(R)-hydroxyeicosatetraenoic acid (15R-HETE). Ser-530 has also been shown to influence the stereochemistry for the addition of oxygen to the prostaglandin product. We determined the crystal structures of S530T murine (mu) COX-2, aspirin-acetylated human (hu) COX-2, and huCOX-2 in complex with salicylate to 1.9, 2.0, and 2.4 Å, respectively. The structures reveal that (1) the acetylated Ser-530 completely blocks access to the hydrophobic groove, (2) the observed binding pose of salicylate is reflective of the enzyme-inhibitor complex prior to acetylation, and (3) the observed Thr-530 rotamer in the S530T muCOX-2 crystal structure does not impede access to the hydrophobic groove. On the basis of these structural observations, along with functional analysis of the S530T/G533V double mutant, we propose a working hypothesis for the generation of 15R-HETE by aspirin-acetylated COX-2. We also observe differential acetylation of COX-2 purified in various detergent systems and nanodiscs, indicating that detergent and lipid binding within the membrane-binding domain of the enzyme alters the rate of the acetylation reaction in vitro.
Figures

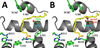

Similar articles
-
Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2.J Lipid Res. 2010 Mar;51(3):575-85. doi: 10.1194/jlr.M001719. Epub 2009 Sep 14. J Lipid Res. 2010. PMID: 19752399 Free PMC article.
-
Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z, 13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. Why acetylated COX-1 does not synthesize 15-(R)-hete.J Biol Chem. 2000 Mar 3;275(9):6586-91. doi: 10.1074/jbc.275.9.6586. J Biol Chem. 2000. PMID: 10692466
-
Arg-513 and Leu-531 Are Key Residues Governing Time-Dependent Inhibition of Cyclooxygenase-2 by Aspirin and Celebrex.Biochemistry. 2019 Sep 24;58(38):3990-4002. doi: 10.1021/acs.biochem.9b00659. Epub 2019 Sep 9. Biochemistry. 2019. PMID: 31469551 Free PMC article.
-
The medicinal chemistry implications of the anticancer effects of aspirin and other NSAIDs.Mini Rev Med Chem. 2003 Aug;3(5):461-70. doi: 10.2174/1389557033488033. Mini Rev Med Chem. 2003. PMID: 12769697 Review.
-
Computer aided drug design approaches to develop cyclooxygenase based novel anti-inflammatory and anti-cancer drugs.Curr Pharm Des. 2007;13(34):3505-17. doi: 10.2174/138161207782794275. Curr Pharm Des. 2007. PMID: 18220787 Review.
Cited by
-
Preparation, COX-2 Inhibition and Anticancer Activity of Sclerotiorin Derivatives.Mar Drugs. 2020 Dec 29;19(1):12. doi: 10.3390/md19010012. Mar Drugs. 2020. PMID: 33383842 Free PMC article.
-
Network Pharmacology Study to Reveal the Potentiality of a Methanol Extract of Caesalpinia sappan L. Wood against Type-2 Diabetes Mellitus.Life (Basel). 2022 Feb 13;12(2):277. doi: 10.3390/life12020277. Life (Basel). 2022. PMID: 35207564 Free PMC article.
-
Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone.J Biol Chem. 2016 Jul 15;291(29):15069-81. doi: 10.1074/jbc.M116.725713. Epub 2016 May 20. J Biol Chem. 2016. PMID: 27226593 Free PMC article.
-
Computational Approaches to Evaluate the Acetylcholinesterase Binding Interaction with Taxifolin for the Management of Alzheimer's Disease.Molecules. 2024 Jan 31;29(3):674. doi: 10.3390/molecules29030674. Molecules. 2024. PMID: 38338420 Free PMC article.
-
Herb-target virtual screening and network pharmacology for prediction of molecular mechanism of Danggui Beimu Kushen Wan for prostate cancer.Sci Rep. 2021 Mar 23;11(1):6656. doi: 10.1038/s41598-021-86141-1. Sci Rep. 2021. PMID: 33758314 Free PMC article.
References
-
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. - PubMed
-
- Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–1441. - PubMed
-
- Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

