Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry
- PMID: 26859324
- PMCID: PMC4775376
- DOI: 10.1021/acs.biochem.5b01378
Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight into the Formation of Products with Reversed Stereochemistry
Abstract
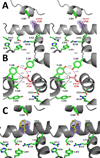
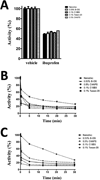
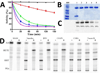
Aspirin and other nonsteroidal anti-inflammatory drugs target the cyclooxygenase enzymes (COX-1 and COX-2) to block the formation of prostaglandins. Aspirin is unique in that it covalently modifies each enzyme by acetylating Ser-530 within the cyclooxygenase active site. Acetylation of COX-1 leads to complete loss of activity, while acetylation of COX-2 results in the generation of the monooxygenated product 15(R)-hydroxyeicosatetraenoic acid (15R-HETE). Ser-530 has also been shown to influence the stereochemistry for the addition of oxygen to the prostaglandin product. We determined the crystal structures of S530T murine (mu) COX-2, aspirin-acetylated human (hu) COX-2, and huCOX-2 in complex with salicylate to 1.9, 2.0, and 2.4 Å, respectively. The structures reveal that (1) the acetylated Ser-530 completely blocks access to the hydrophobic groove, (2) the observed binding pose of salicylate is reflective of the enzyme-inhibitor complex prior to acetylation, and (3) the observed Thr-530 rotamer in the S530T muCOX-2 crystal structure does not impede access to the hydrophobic groove. On the basis of these structural observations, along with functional analysis of the S530T/G533V double mutant, we propose a working hypothesis for the generation of 15R-HETE by aspirin-acetylated COX-2. We also observe differential acetylation of COX-2 purified in various detergent systems and nanodiscs, indicating that detergent and lipid binding within the membrane-binding domain of the enzyme alters the rate of the acetylation reaction in vitro.
Figures

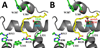

References
-
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. - PubMed
-
- Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–1441. - PubMed
-
- Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

