Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis
- PMID: 26859364
- PMCID: PMC4899224
- DOI: 10.1038/pr.2016.18
Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis
Abstract
Background: Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis of premature infants. Based on our recent findings of increased Smad7 expression in surgically resected bowel affected by NEC, we hypothesized that NEC macrophages undergo inflammatory activation because increased Smad7 expression renders these cells resistant to normal, gut-specific, transforming growth factor (TGF)-β-mediated suppression of inflammatory pathways.
Methods: We used surgically resected human NEC tissue, murine models of NEC-like injury, bone marrow-derived and intestinal macrophages, and RAW264.7 cells. Smad7 and IκB kinase-beta (IKK-β) were measured by quantitative PCR, western blots, and immunohistochemistry. Promoter activation was confirmed in luciferase reporter and chromatin immunoprecipitation assays.
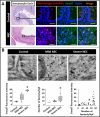
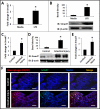
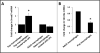
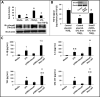
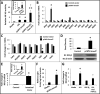
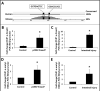
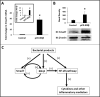
Results: NEC macrophages showed increased Smad7 expression, particularly in areas with severe tissue damage and high bacterial load. Lipopolysaccharide-induced Smad7 expression suppressed TGF-β signaling and augmented nuclear factor-kappa B (NF-κB) activation and cytokine production in macrophages. Smad7-mediated NF-κB activation was likely mediated via increased expression of IKK-β, which, further increased Smad7 expression in a feed-forward loop. We show that Smad7 induced IKK-β expression through direct binding to the IKK-β promoter and its transcriptional activation.
Conclusion: Smad7 expression in NEC macrophages interrupts TGF-β signaling and promotes NF-κB-mediated inflammatory signaling in these cells through increased expression of IKK-β.
Figures
Similar articles
-
Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in baboon necrotizing enterocolitis.Am J Physiol Gastrointest Liver Physiol. 2013 Jan 15;304(2):G167-80. doi: 10.1152/ajpgi.00141.2012. Epub 2012 Nov 15. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23154975 Free PMC article.
-
TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine.Gastroenterology. 2011 Jan;140(1):242-53. doi: 10.1053/j.gastro.2010.09.043. Epub 2010 Sep 24. Gastroenterology. 2011. PMID: 20875417 Free PMC article.
-
Blocking NF-κB Activation in Ly6c+ Monocytes Attenuates Necrotizing Enterocolitis.Am J Pathol. 2019 Mar;189(3):604-618. doi: 10.1016/j.ajpath.2018.11.015. Epub 2018 Dec 27. Am J Pathol. 2019. PMID: 30593820 Free PMC article.
-
Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns.Int J Biochem Cell Biol. 2013 Aug;45(8):1730-47. doi: 10.1016/j.biocel.2013.04.028. Epub 2013 May 6. Int J Biochem Cell Biol. 2013. PMID: 23660296 Review.
-
Expression and function of Smad7 in autoimmune and inflammatory diseases.J Mol Med (Berl). 2021 Sep;99(9):1209-1220. doi: 10.1007/s00109-021-02083-1. Epub 2021 May 31. J Mol Med (Berl). 2021. PMID: 34059951 Free PMC article. Review.
Cited by
-
Integrated analysis of a lncRNA‑mRNA network reveals a potential mechanism underlying necrotizing enterocolitis.Mol Med Rep. 2020 Jul;22(1):423-435. doi: 10.3892/mmr.2020.11083. Epub 2020 Apr 21. Mol Med Rep. 2020. PMID: 32319640 Free PMC article.
-
Severe neonatal anemia increases intestinal permeability by disrupting epithelial adherens junctions.Am J Physiol Gastrointest Liver Physiol. 2020 Apr 1;318(4):G705-G716. doi: 10.1152/ajpgi.00324.2019. Epub 2020 Feb 24. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32090604 Free PMC article.
-
The Role of Glycosaminoglycans in Protection from Neonatal Necrotizing Enterocolitis: A Narrative Review.Nutrients. 2020 Feb 20;12(2):546. doi: 10.3390/nu12020546. Nutrients. 2020. PMID: 32093194 Free PMC article. Review.
-
Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria.Pediatr Res. 2020 Jan;87(2):235-248. doi: 10.1038/s41390-019-0482-9. Epub 2019 Jun 25. Pediatr Res. 2020. PMID: 31238334 Free PMC article. Review.
-
Extracellular Nicotinamide Phosphoribosyltransferase Is a Therapeutic Target in Experimental Necrotizing Enterocolitis.Biomedicines. 2024 Apr 28;12(5):970. doi: 10.3390/biomedicines12050970. Biomedicines. 2024. PMID: 38790933 Free PMC article.
References
-
- Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990;117:S6–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

