Isotope effect analyses provide evidence for an altered transition state for RNA 2'-O-transphosphorylation catalyzed by Zn(2+)
- PMID: 26859380
- PMCID: PMC4916458
- DOI: 10.1039/c5cc10212j
Isotope effect analyses provide evidence for an altered transition state for RNA 2'-O-transphosphorylation catalyzed by Zn(2+)
Abstract
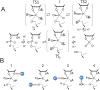
Solvent D2O and (18)O kinetic isotope effects on RNA 2'-O-transphosphorylation catalyzed by Zn(2+) demonstrate an altered transition state relative to specific base catalysis. A recent model from DFT calculations involving inner sphere coordination to the non-bridging and leaving group oxygens is consistent with the data.
Figures


References
-
- Wilcox DE. Chem Rev. 1996;96:2435–2458. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

