Surfactant Lipids at the Host-Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease
- PMID: 26859434
- PMCID: PMC4942198
- DOI: 10.1165/rcmb.2016-0011PS
Surfactant Lipids at the Host-Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease
Abstract
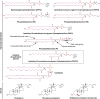
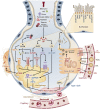
The lipid composition of pulmonary surfactant is unlike that of any other body fluid. This extracellular lipid reservoir is also uniquely susceptible by virtue of its direct and continuous exposure to environmental oxidants, inflammatory agents, and pathogens. Historically, the greatest attention has been focused on those biophysical features of surfactant that serve to reduce surface tension at the air-liquid interface. More recently, surfactant lipids have also been recognized as bioactive molecules that maintain immune quiescence in the lung but can also be remodeled by the inhaled environment into neolipids that mediate key roles in inflammation, immunity, and fibrosis. This review focuses on the roles in inflammatory and infectious lung disease of two classes of native surfactant lipids, glycerophospholipids and sterols, and their corresponding oxidized species, oxidized glycerophospholipids and oxysterols. We highlight evidence that surfactant composition is sensitive to circulating lipoproteins and that the lipid milieu of the alveolus should thus be recognized as susceptible to diet and common systemic metabolic disorders. We also discuss intriguing evidence suggesting that oxidized surfactant lipids may represent an evolutionary link between immunity and tissue homeostasis that arose in the primordial lung. Taken together, the emerging picture is one in which the unique environmental susceptibility of the lung, together with its unique extracellular lipid requirements, may have made this organ both an evolutionary hub and an engine for lipid-immune cross-talk.
Keywords: cholesterol; innate immunity; lung; phospholipid; surfactant.
Figures
Similar articles
-
Pulmonary surfactants and their role in pathophysiology of lung disorders.Indian J Exp Biol. 2013 Jan;51(1):5-22. Indian J Exp Biol. 2013. PMID: 23441475 Review.
-
Decreased surfactant lipids correlate with lung function in chronic obstructive pulmonary disease (COPD).PLoS One. 2020 Feb 6;15(2):e0228279. doi: 10.1371/journal.pone.0228279. eCollection 2020. PLoS One. 2020. PMID: 32027677 Free PMC article.
-
Endogenous LXR signaling controls pulmonary surfactant homeostasis and prevents lung inflammation.Cell Mol Life Sci. 2024 Jul 6;81(1):287. doi: 10.1007/s00018-024-05310-3. Cell Mol Life Sci. 2024. PMID: 38970705 Free PMC article.
-
Clearance of surfactant lipids by neutrophils and macrophages isolated from the acutely inflamed lung.Am J Physiol Lung Cell Mol Physiol. 2002 Feb;282(2):L330-9. doi: 10.1152/ajplung.00190.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 11792638
-
Role of lipid ordered/disordered phase coexistence in pulmonary surfactant function.Biochim Biophys Acta. 2012 Nov;1818(11):2550-62. doi: 10.1016/j.bbamem.2012.05.024. Epub 2012 May 31. Biochim Biophys Acta. 2012. PMID: 22659676 Review.
Cited by
-
Lung lipids associated with smoking and ECIG use in a cross-sectional study and clinical trial.Respir Res. 2025 May 20;26(1):193. doi: 10.1186/s12931-025-03267-w. Respir Res. 2025. PMID: 40394619 Free PMC article. Clinical Trial.
-
Update on the spider and the fly: An extended commentary on "Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-Oxidase activation".Free Radic Biol Med. 2016 Jul;96:462-4. doi: 10.1016/j.freeradbiomed.2016.03.034. Epub 2016 Apr 1. Free Radic Biol Med. 2016. PMID: 27040582 Free PMC article. No abstract available.
-
Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work.Int J Mol Sci. 2020 Apr 28;21(9):3104. doi: 10.3390/ijms21093104. Int J Mol Sci. 2020. PMID: 32354030 Free PMC article. Review.
-
CES1 Releases Oxylipins from Oxidized Triacylglycerol (oxTAG) and Regulates Macrophage oxTAG/TAG Accumulation and PGE2/IL-1β Production.ACS Chem Biol. 2023 Jul 21;18(7):1564-1581. doi: 10.1021/acschembio.3c00194. Epub 2023 Jun 22. ACS Chem Biol. 2023. PMID: 37348046 Free PMC article.
-
Aqueous extract of Platycodon grandiflorus attenuates lipopolysaccharide-induced apoptosis and inflammatory cell infiltration in mouse lungs by inhibiting PI3K/Akt signaling.Chin Med. 2023 Apr 4;18(1):36. doi: 10.1186/s13020-023-00721-z. Chin Med. 2023. PMID: 37016413 Free PMC article.
References
-
- Postle AD, Heeley EL, Wilton DC. A comparison of the molecular species compositions of mammalian lung surfactant phospholipids. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:65–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

